Key Points
-
In autosomal dominant polycystic kidney disease (ADPKD), renal cyst formation begins in utero and continues throughout life
-
Renal cysts originate in tubules and contribute to the development of renal insufficiency
-
Individual renal cysts progressively expand at a constant rate that can differ widely from the growth rate of neighbouring cysts
-
Cysts disrupt the renal ultrastructure in the early stages of ADPKD and cause renin-dependent hypertension, albuminuria, interstitial inflammation, fibrosis, and the destruction of functioning nephrons.
-
Evidence indicates that the annual rate of kidney growth in ADPKD is inversely linked to the decline in glomerular filtration rate so can be used to predict future decline in glomerular function
-
Longitudinal studies in thousands of patients have provided evidence to validate the use of total kidney volume as a prognostic marker and as a potential indicator of treatment efficacy in ADPKD
Abstract
The rate at which autosomal dominant polycystic kidney disease (ADPKD) progresses to end-stage renal disease varies widely and is determined by genetic and non-genetic factors. The ability to determine the prognosis of children and young adults with ADPKD is important for the effective life-long management of the disease and to enable the efficacy of emerging therapies to be determined. Total kidney volume (TKV) reflects the sum volume of hundreds of individual cysts with potentially devastating effects on renal function. The sequential measurement of TKV has been advanced as a dynamic biomarker of disease progression, yet doubt remains among nephrologists and regulatory agencies as to its usefulness. Here, we review the mechanisms that lead to an increase in TKV in ADPKD, and examine the evidence supporting the conclusion that TKV provides a metric of disease progression that can be used to assess the efficacy of potential therapeutic regimens in children and adults with ADPKD. Moreover, we propose that TKV can be used to monitor treatment efficacy in patients with normal levels of renal function, before the pathologic processes of ADPKD cause extensive fibrosis and irreversible loss of functioning renal tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grantham, J. J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 359, 1477–1485 (2008).
Franz, K. A. & Reubi, F. C. Rate of functional deterioration in polycystic kidney disease. Kidney Int. 23, 526–529 (1983).
Dalgaard, O. Z. Bilateral polycystic disease of the kidneys; a follow-up of 284 patients and their families. Dan. Med. Bull. 4, 128–133 (1957).
Levine, E. & Grantham, J. J. The role of computed tomography in the evaluation of adult polycystic kidney disease. Am. J. Kidney Dis. 1, 99–105 (1981).
Gabow, P. A., Ikle, D. W. & Holmes, J. H. Polycystic kidney disease: prospective analysis of nonazotemic patients and family members. Ann. Intern. Med. 101, 238–247 (1984).
Grantham, J. J., Geiser, J. L. & Evan, A. P. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 31, 1145–1152 (1987).
Torres, V. E. & Harris, P. C. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 76, 149–168 (2009).
Wallace, D. P. Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta 1812, 1291–1300 (2011).
Gattone, V. H., 2nd, Wang, X., Harris, P. C. & Torres, V. E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 9, 1323–1326 (2003).
Wang, X., Wu, Y., Ward, C. J., Harris, P. C. & Torres, V. E. Vasopressin directly regulates cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 102–108 (2008).
Meijer, E. et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 361–368 (2011).
Gardner, K. D. Jr & Evan, A. P. Renal cystic disease induced by diphenylthiazole. Kidney Int. 24, 43–52 (1983).
Gardner, K. D. Jr., Evan, A. P. & Reed, W. P. Accelerated renal cyst development in deconditioned germ-free rats. Kidney Int. 29, 1116–1123 (1986).
Gardner, K. D. Jr et al. Endotoxin provocation of experimental renal cystic disease. Kidney Int. 32, 329–334 (1987).
Grantham, J. J. Acquired cystic kidney disease. Kidney Int. 40, 143–152 (1991).
Carone, F. A. & Kanwar, Y. Tubular cell and matrix changes in renal cystic disease. Contrib. Nephrol. 101, 1–6 (1993).
Torres, V. E. & Harris, P. C. Polycystic kidney disease in 2011: connecting the dots toward a polycystic kidney disease therapy. Nat. Rev. Nephrol. 8, 66–68 (2012).
Ong, A. C. & Harris, P. C. A polycystin-centric view of cyst formation and disease: the polycystins revisited. Kidney Int. 88, 699–710 (2015).
Ecder, T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr. Hypertens. Rev. 9, 2–11 (2013).
Nowak, K. L., Farmer, H., Cadnapaphornchai, M. A., Gitomer, B. & Chonchol, M. Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfw013 (2016).
Merta, M. et al. Role of endothelin and nitric oxide in the pathogenesis of arterial hypertension in autosomal dominant polycystic kidney disease. Physiol. Res. 52, 433–437 (2003).
Devuyst, O. & Torres, V. E. Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 22, 459–470 (2013).
Bichet, D. G. A defect in vasopressin secretion in autosomal dominant polycystic kidney disease. Kidney Int. 82, 1051–1053 (2012).
Nagao, S. et al. Renal activation of extracellular signal-regulated kinase in rats with autosomal-dominant polycystic kidney disease. Kidney Int. 63, 427–437 (2003).
Zheng, D. et al. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 14, 2588–2595 (2003).
Meijer, E. et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am. J. Kidney Dis. 56, 883–895 (2010).
Azurmendi, P. J. et al. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol. Dial. Transplant. 24, 2458–2463 (2009).
Karihaloo, A. et al. Macrophages promote cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 22, 1809–1814 (2011).
Swenson-Fields, K. I. et al. Macrophages promote polycystic kidney disease progression. Kidney Int. 83, 855–864 (2013).
Cadnapaphornchai, M. A., McFann, K., Strain, J. D., Masoumi, A. & Schrier, R. W. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 74, 1192–1196 (2008).
Seeman, T. et al. Blood pressure and renal function in autosomal dominant polycystic kidney disease. Pediatr. Nephrol. 11, 592–596 (1997).
Fonseca, J. M. et al. Renal cyst growth is the main determinant for hypertension and concentrating deficit in Pkd1-deficient mice. Kidney Int. 85, 1137–1150 (2014).
Saigusa, T. et al. Activation of the intrarenal renin-angiotensin-system in murine polycystic kidney disease. Physiol. Rep. 3, e12405 (2015).
Grantham, J. J., Cook, L. T., Wetzel, L. H., Cadnapaphornchai, M. A. & Bae, K. T. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin. J. Am. Soc. Nephrol. 5, 889–896 (2010).
Harris, P. C. et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 17, 3013–3019 (2006).
Helal, I. et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 28, 380–385 (2013).
Klawitter, J. et al. Bioactive lipid mediators in polycystic kidney disease. J. Lipid Res. 55, 1139–1149 (2013).
Kistler, A. D. et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS ONE 8, e53016 (2013).
Grantham, J. J. et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 73, 108–116 (2008).
Heyer, C. M. et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 2872–2884 (2016).
Hwang, Y. H. et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 1861–1868 (2015).
Harskamp, L. R. et al. Urinary EGF receptor ligand excretion in patients with autosomal dominant polycystic kidney disease and response to tolvaptan. Clin. J. Am. Soc. Nephrol. 10, 1749–1756 (2015).
Liu, C., Zhang, Y., Yuan, L., Fu, L. & Mei, C. Rosiglitazone inhibits insulin-like growth factor1-induced polycystic kidney disease cell growth and p70S6 kinase activation. Mol. Med. Rep. 8, 861–864 (2013).
Galarreta, C. I. et al. Tubular obstruction leads to progressive proximal tubular injury and atubular glomeruli in polycystic kidney disease. Am. J. Pathol. 184, 1957–1966 (2014).
Hayslett, J. P., Kashgarian, M. & Epstein, F. H. Functional correlates of compensatory renal hypertrophy. J. Clin. Invest. 47, 774–799 (1968).
Ibrahim, H. N. et al. Long-term consequences of kidney donation. N. Engl. J. Med. 360, 459–469 (2009).
Bricker, N. S. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N. Engl. J. Med. 286, 1093–1099 (1972).
Gansevoort, R. T. et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfv422 (2015).
Torres, V. E. et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2, 112–120 (2007).
King, B. F. et al. Quantification and longitudinal trends of kidney, renal cyst, and renal parenchyma volumes in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 11, 1505–1511 (2000).
Sise, C. et al. Volumetric determination of progression in autosomal dominant polycystic kidney disease by computed tomography. Kidney Int. 58, 2492–2501 (2000).
Grantham, J. J., Chapman, A. B. & Torres, V. E. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 1, 148–157 (2006).
Grantham, J. J. et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 354, 2122–2130 (2006).
Chapman, A. B. et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 7, 479–486 (2012).
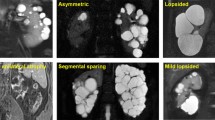
Irazabal, M. V. et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 26, 160–172 (2015).
Bhutani, H. et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 88, 146–151 (2015).
Perrone, R. D. et al. Qualification of total kidney volume as a prognostic biomarker for use in clinical trials evaluating patients with polycystic kidney disease. Am. J. Kidney Dis. 63, B119 (2014).
U.S. Department of Health & Human Services, Food & Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf (2014).
Bonventre, J. V. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol. Dial. Transplant. 24, 3265–3268 (2009).
Trachtman, H. et al. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr. Nephrol. 21, 989–994 (2006).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Torres, V. E., Gansevoort, R. T. & Czerwiec, F. S. Tolvaptan in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 368, 1259 (2013).
Grantham, J. J. et al. Tolvaptan suppresses monocyte chemotactic protein-1 in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfw060 (2016).
Schrier, R. W. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 (2014).
Caroli, A. et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 382, 1485–1495 (2013).
Meijer, E. et al. Rationale and design of the DIPAK 1 study: a randomized controlled clinical trial assessing the efficacy of lanreotide to Halt disease progression in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 63, 446–455 (2014).
Cadnapaphornchai, M. A. et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 9, 889–896 (2014).
Torres, V. E. & Harris, P. C. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J. Intern. Med. 261, 17–31 (2007).
Ong, A. C., Devuyst, O., Knebelmann, B., Walz, G. & ERA-EDTA Working Group for Inherited Kidney Diseases Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385, 1993–2002 (2015).
Torres, V. E. et al. Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 trial. Clin. J. Am. Soc. Nephrol. 11, 803–811 (2016).
Irazabal, M. V. et al. Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the HALT-PKD clinical trial. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfw294 (2016).
Grantham, J. J. Rationale for early treatment of polycystic kidney disease. Pediatr. Nephrol. 30, 1053–1062 (2015).
Cardiovascular and Renal Drugs Advisory Committee Tolvaptan: slowing progression of Autosomal Dominant Polycystic Kidney Disease (ADPKD) http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM364583.pdf (2013).
Lee, K. R., Grantham, J. J. & Cook, P. N. Volume estimation from computed tomography for polycystic kidney disease. Automedica 4, 75–79 (1981).
Thomsen, H. S., Madsen, J. K., Thaysen, J. H. & Damgaard-Petersen, K. Volume of polycystic kidneys during reduction of renal function. Urol. Radiol 3, 85–89 (1981).
Ruggenenti, P. et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 68, 206–216 (2005).
Antiga, L. et al. Computed tomography evaluation of autosomal dominant polycystic kidney disease progression: a progress report. Clin. J. Am. Soc. Nephrol. 1, 754–760 (2006).
Cadnapaphornchai, M. A., Masoumi, A., Strain, J. D., McFann, K. & Schrier, R. W. Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin. J. Am. Soc. Nephrol. 6, 369–376 (2011).
Tokiwa, S., Muto, S., China, T. & Horie, S. The relationship between renal volume and renal function in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 15, 539–545 (2011).
Higashihara, E. et al. Renal disease progression in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 16, 622–628 (2012).
Chen, D. et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS ONE 9, e92232 (2014).
Author information
Authors and Affiliations
Contributions
J.J.G and V.E.T contributed equally to researching data for the article, discussion of the content, and revising or editing the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J.J.G and V.E.T have consulted for and received research grants from Otsuka Pharmaceutical Development and Commercialization, Inc.
Glossary
- Planar cell polarity
-
Planar polarization refers to the spatial orientation of a group of cells, whereby the cells and their structures are orientated within the same plane.
- Desmopressin
-
A synthetic and highly specific vasopressin V2 agonist.
Rights and permissions
About this article
Cite this article
Grantham, J., Torres, V. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 12, 667–677 (2016). https://doi.org/10.1038/nrneph.2016.135
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.135
This article is cited by
-
Non-contrast low-dose CT can be used for volumetry of ADPKD
BMC Nephrology (2023)
-
Convolutional neural network-based kidney volume estimation from low-dose unenhanced computed tomography scans
BMC Medical Imaging (2023)
-
Diffusion magnetic resonance imaging for kidney cyst volume quantification and non-cystic tissue characterisation in ADPKD
European Radiology (2023)
-
Estimating risk of rapid disease progression in pediatric patients with autosomal dominant polycystic kidney disease: a randomized trial of tolvaptan
Pediatric Nephrology (2023)
-
Kidney epithelial cells are active mechano-biological fluid pumps
Nature Communications (2022)