Key Points
-
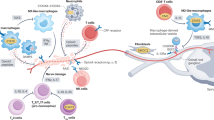
Local and recruited immunocompetent cells (such as microglia, astrocytes, endothelial cells, perivascular macrophages and T cells) in the central nervous system (CNS) detect neurotransmitters, chemokines and endogenous danger signals that are released by lesioned or diseased sensory neurons.
-
Immunocompetent cells in the central nervous system subsequently release cytokines, chemokines, prostaglandins, neurotrophic factors and reactive oxygen species that dysregulate synaptic transmission leading to amplification of nociceptive signalling.
-
Other facets of the immune response to neuronal lesion and disease are gaining recognition, including the necessity of pro-inflammatory mediators for repair, and regulation of pro-inflammatory responses by anti-inflammatory mediators.
-
Hence, the most successful treatment approaches targeting the immune system will probably integrate basic science understanding of nuanced immune responses.
-
Despite there being only indirect evidence for a CNS immune component to chronic pain in humans, immune-targeted therapies are showing early signs of success in treating such pain.
Abstract
Reciprocal signalling between immunocompetent cells in the central nervous system (CNS) has emerged as a key phenomenon underpinning pathological and chronic pain mechanisms. Neuronal excitability can be powerfully enhanced both by classical neurotransmitters derived from neurons, and by immune mediators released from CNS-resident microglia and astrocytes, and from infiltrating cells such as T cells. In this Review, we discuss the current understanding of the contribution of central immune mechanisms to pathological pain, and how the heterogeneous immune functions of different cells in the CNS could be harnessed to develop new therapeutics for pain control. Given the prevalence of chronic pain and the incomplete efficacy of current drugs — which focus on suppressing aberrant neuronal activity — new strategies to manipulate neuroimmune pain transmission hold considerable promise.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Maier, S. F., Wiertelak, E. P., Martin, D. & Watkins, L. R. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 623, 321–324 (1993).
Ferreira, S. H., Lorenzetti, B. B., Bristow, A. F. & Poole, S. Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 334, 698–700 (1988).
Watkins, L. R. et al. Characterization of cytokine-induced hyperalgesia. Brain Res. 654, 15–26 (1994).
Bianchi, M., Sacerdote, P., Ricciardi-Castagnoli, P., Mantegazza, P. & Panerai, A. E. Central effects of tumor necrosis factor alpha and interleukin-1 alpha on nociceptive thresholds and spontaneous locomotor activity. Neurosci. Lett. 148, 76–80 (1992).
Watkins, L. R., Maier, S. F. & Goehler, L. E. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 63, 289–302 (1995).
Garrison, C. J., Dougherty, P. M., Kajander, K. C. & Carlton, S. M. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 565, 1–7 (1991).
Svensson, M. et al. The response of central glia to peripheral nerve injury. Brain Res. Bull. 30, 499–506 (1993).
Watkins, L. R., Martin, D., Ulrich, P., Tracey, K. J. & Maier, S. F. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain 71, 225–235 (1997).
Meller, S. T., Dykstra, C., Grzybycki, D., Murphy, S. & Gebhart, G. F. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 33, 1471–1478 (1994).
Ledeboer, A. et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115, 71–83 (2005).
Milligan, E. D. et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 21, 2808–2819 (2001). The first demonstration that spinal microgliosis is sufficient to induce nociceptive hypersensitivity via production and release of pro-inflammatory cytokines.
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Ossipov, M. H., Dussor, G. O. & Porreca, F. Central modulation of pain. J. Clin. Invest. 120, 3779–3787 (2010).
Pizzo, P. A. & Clark, N. M. Alleviating suffering 101-pain relief in the United States. N. Engl. J. Med. 366, 197–199 (2012).
Latremoliere, A. & Woolf, C. J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926 (2009). An excellent review of the neuronal changes implicated in the creation and maintenance of exaggerated pain states.
Scholz, J. & Woolf, C. J. The neuropathic pain triad: neurons, immune cells and glia. Nature Neurosci. 10, 1361–1368 (2007).
Araque, A. & Navarrete, M. Glial cells in neuronal network function. Phil. Trans. R. Soc. B 365, 2375–2381 (2010).
Hanisch, U. K. Functional diversity of microglia - how heterogeneous are they to begin with? Front. Cell Neurosci. 7, 65 (2013).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
Sofroniew, M. V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 (2009).
Ulmann, L. et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 28, 11263–11268 (2008).
Grace, P. M., Rolan, P. E. & Hutchinson, M. R. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav. Immun. 25, 1322–1332 (2011).
Zhang, J. et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J. Neurosci. 27, 12396–12406 (2007).
Costigan, M. et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 29, 14415–14422 (2009).
Leger, T., Grist, J., D'Acquisto, F., Clark, A. K. & Malcangio, M. Glatiramer acetate attenuates neuropathic allodynia through modulation of adaptive immune cells. J. Neuroimmunol. 234, 19–26 (2011).
Meng, X. et al. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 154, 294–305 (2013).
Tsuda, M. et al. IFNγ receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl Acad. Sci. USA 106, 8032–8037 (2009).
Austin, P. J., Kim, C. F., Perera, C. J. & Moalem-Taylor, G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 153, 1916–1931 (2012).
Taoka, Y. et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience 79, 1177–1182 (1997).
Sweitzer, S. M., Hickey, W. F., Rutkowski, M. D., Pahl, J. L. & DeLeo, J. A. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain 100, 163–170 (2002).
Kwok, Y. H. et al. TLR 2 and 4 responsiveness from isolated peripheral blood mononuclear cells from rats and humans as potential chronic pain biomarkers. PLoS ONE 8, e77799 (2013).
Grace, P. M. et al. Harnessing pain heterogeneity and RNA transcriptome to identify blood-based pain biomarkers: a novel correlational study design and bioinformatics approach in a graded chronic constriction injury model. J. Neurochem. 122, 976–994 (2012).
Kwok, Y. H., Hutchinson, M. R., Gentgall, M. G. & Rolan, P. E. Increased responsiveness of peripheral blood mononuclear cells to in vitro TLR 2, 4 and 7 ligand stimulation in chronic pain patients. PLoS ONE 7, e44232 (2012).
Hutchinson, M. R., La Vincente, S. F. & Somogyi, A. A. In vitro opioid induced proliferation of peripheral blood immune cells correlates with in vivo cold pressor pain tolerance in humans: a biological marker of pain tolerance. Pain 110, 751–755 (2004).
Tian, L., Rauvala, H. & Gahmberg, C. G. Neuronal regulation of immune responses in the central nervous system. Trends Immunol. 30, 91–99 (2009).
Hoarau, J. J. et al. Activation and control of CNS innate immune responses in health and diseases: a balancing act finely tuned by neuroimmune regulators (NIReg). CNS Neurol. Disord. Drug Targets 10, 25–43 (2011).
Coull, J. A. et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003).
Coull, J. A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005). The first report to identify a mechanism for glial-mediated enhancement of pain signalling.
Tsuda, M. et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783 (2003).
Ji, R. R., Berta, T. & Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 154 (Suppl. 1), 10–28 (2013).
Milligan, E. D. & Watkins, L. R. Pathological and protective roles of glia in chronic pain. Nature Rev. Neurosci. 10, 23–36 (2009).
Kim, D. et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 282, 14975–14983 (2007).
Wen, Y. R. et al. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology 107, 312–321 (2007).
Suter, M. R., Berta, T., Gao, Y. J., Decosterd, I. & Ji, R. R. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain 5, 53 (2009).
Kawasaki, Y. et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nature Med. 14, 331–336 (2008).
Calvo, M. et al. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J. Neurosci. 30, 5437–5450 (2010).
Abbadie, C. et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl Acad. Sci. USA 100, 7947–7952 (2003).
Van Steenwinckel, J. et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J. Neurosci. 31, 5865–5875 (2011).
Dansereau, M. A. et al. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J. Neurochem. 106, 757–769 (2008).
Clark, A. K. et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl Acad. Sci. USA 104, 10655–10660 (2007).
Milligan, E. D. et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur. J. Neurosci. 20, 2294–2302 (2004).
Staniland, A. A. et al. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J. Neurochem. 114, 1143–1157 (2010).
Clark, A. K., Wodarski, R., Guida, F., Sasso, O. & Malcangio, M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 58, 1710–1726 (2010).
Gao, Y. J. & Ji, R. R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 126, 56–68 (2010).
Wolf, Y., Yona, S., Kim, K. W. & Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell Neurosci. 7, 26 (2013).
Zhao, P., Waxman, S. G. & Hains, B. C. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J. Neurosci. 27, 8893–8902 (2007).
Biber, K. et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 30, 1864–1873 (2011).
Inoue, K. & Tsuda, M. Purinergic systems, neuropathic pain and the role of microglia. Exp. Neurol. 234, 293–301 (2012). A comprehensive review of the role of purinergic receptor signalling in pain.
Friedle, S. A., Curet, M. A. & Watters, J. J. Recent patents on novel P2X7 receptor antagonists and their potential for reducing central nervous system inflammation. Recent Pat. CNS Drug Discov. 5, 35–45 (2010).
Bardoni, R., Goldstein, P. A., Lee, C. J., Gu, J. G. & MacDermott, A. B. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 17, 5297–5304 (1997).
Sorge, R. E. et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nature Med. 18, 595–599 (2012).
He, W. J. et al. Spinal P2X7 receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav. Brain Res. 226, 163–170 (2012).
Chu, Y. X., Zhang, Y., Zhang, Y. Q. & Zhao, Z. Q. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav. Immun. 24, 1176–1189 (2010).
Yamamoto, K. et al. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 279, H285–H292 (2000).
Nicke, A. et al. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J. Biol. Chem. 284, 25813–25822 (2009).
Clark, A. K. et al. P2X7-dependent release of interleukin-1β and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 30, 573–582 (2010).
Kobayashi, K. et al. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J. Neurosci. 28, 2892–2902 (2008).
Tozaki-Saitoh, H. et al. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J. Neurosci. 28, 4949–4956 (2008).
Saijo, K., Crotti, A. & Glass, C. K. Regulation of microglia activation and deactivation by nuclear receptors. Glia 61, 104–111 (2013).
Nicotra, L., Loram, L. C., Watkins, L. R. & Hutchinson, M. R. Toll-like receptors in chronic pain. Exp. Neurol. 234, 316–329 (2012). A comprehensive review of the role of TLR signalling in pain.
Kuang, X. et al. Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur. J. Pharmacol. 676, 51–56 (2012).
Ren, P. C. et al. High-mobility group box contributes to mechanical allodynia and spinal astrocytic activation in a mouse model of type 2 diabetes. Brain Res. Bull. 88, 332–337 (2012).
Tong, W. et al. Spinal high-mobility group box 1 contributes to mechanical allodynia in a rat model of bone cancer pain. Biochem. Biophys. Res. Commun. 395, 572–576 (2010).
Hutchinson, M. R. et al. Evidence for a role of heat shock protein-90 in toll like receptor 4 mediated pain enhancement in rats. Neuroscience 164, 1821–1832 (2009).
Zou, W. et al. Identification of differentially expressed proteins in the spinal cord of neuropathic pain models with PKCγ silence by proteomic analysis. Brain Res. 1440, 34–46 (2012).
Tanga, F. Y., Nutile-McMenemy, N. & DeLeo, J. A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl Acad. Sci. USA 102, 5856–5861 (2005).
Cao, L., Tanga, F. Y. & Deleo, J. A. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience 158, 896–903 (2009).
Bettoni, I. et al. Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia 56, 1312–1319 (2008).
Hutchinson, M. R. et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci. 28, 20–29 (2008).
Kropf, P. et al. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 72, 1920–1928 (2004).
Nicotra, L. L. et al. in Australasian Neuroscience Society Annual Meeting. The role of oestrogenic innate immune priming in exacerbated female pain. Abstr. 148 (Adelaide, 2014).
Vontell, R. et al. Toll-like receptor 3 expression in glia and neurons alters in response to white matter injury in preterm infants. Dev. Neurosci. 35, 130–139 (2013).
Leow-Dyke, S. et al. Neuronal Toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J. Neuroinflammation 9, 230 (2012).
Liu, H. Y. et al. TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 Pathway. J. Neurosci. 33, 11479–11493 (2013).
Acosta, C. & Davies, A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J. Neurosci. Res. 86, 1077–1086 (2008).
Li, Y. Y. et al. Src/p38 MAPK pathway in spinal microglia is involved in mechanical allodynia induced by peri-sciatic administration of recombinant rat TNF-α. Brain Res. Bull. 96, 54–61 (2013).
Katsura, H. et al. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J. Neurosci. 26, 8680–8690 (2006).
Zhong, Y. et al. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-α. Brain Behav. Immun. 24, 874–880 (2010).
Milligan, E. D. et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol. 2, 293–308 (2006).
Chiu, I. M. et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 (2013).
Kawasaki, Y., Zhang, L., Cheng, J. K. & Ji, R. R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28, 5189–5194 (2008).
Gao, Y. J. et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J. Neurosci. 29, 4096–4108 (2009).
Zhang, H., Nei, H. & Dougherty, P. M. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-α. J. Neurosci. 30, 12844–12855 (2010).
Nishio, N. et al. Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating TRPA1 and TRPV1 channels. Neuroscience 247, 201–212 (2013).
Yan, X. & Weng, H. R. Endogenous interleukin-1β in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic NMDA receptors. J Biol Chem. 288, 30544–30557 (2013).
Medvedeva, Y. V., Kim, M. S. & Usachev, Y. M. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J. Neurosci. 28, 5295–5311 (2008).
Park, C. K. et al. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J. Neurosci. 31, 15072–15085 (2011).
Stellwagen, D., Beattie, E. C., Seo, J. Y. & Malenka, R. C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci. 25, 3219–3228 (2005).
Stellwagen, D. & Malenka, R. C. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059 (2006).
Zhang, R. X. et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 135, 232–239 (2008).
Gao, X., Kim, H. K., Chung, J. M. & Chung, K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 131, 262–271 (2007).
Viviani, B. et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 23, 8692–8700 (2003).
Zhao, P., Waxman, S. G. & Hains, B. C. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J. Neurosci. 27, 2357–2368 (2007).
Zhang, Z. J., Cao, D. L., Zhang, X., Ji, R. R. & Gao, Y. J. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 154, 2185–2197 (2013).
Xin, W. J., Weng, H. R. & Dougherty, P. M. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Mol. Pain 5, 15 (2009).
Ramos, K. M. et al. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience 169, 1888–1900 (2010).
Harvey, R. J. et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 (2004).
Gosselin, R. D. et al. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J. Neurochem. 95, 1023–1034 (2005).
Vikman, K. S., Duggan, A. W. & Siddall, P. J. Interferon-γ induced disruption of GABAergic inhibition in the spinal dorsal horn in vivo. Pain 133, 18–28 (2007).
Keller, A. F., Beggs, S., Salter, M. W. & De Koninck, Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain 3, 27 (2007).
Willemen, H. L. et al. MicroRNA-124 as a novel treatment for persistent hyperalgesia. J. Neuroinflamm. 9, 143 (2012).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009). A comprehensive review of microglia that covers the diverse phenotypes that arise in response to a range of stimulatory signals.
Loram, L. C. et al. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav. Immun. 33, 112–122 (2013).
Loram, L. C. et al. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J. Neurosci. 29, 14015–14025 (2009).
Sabat, R. et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 21, 331–344 (2010).
Luzina, I. G. et al. Regulation of inflammation by interleukin-4: a review of “alternatives”. J. Leukoc. Biol. 92, 753–764 (2012).
Ndong, C., Landry, R. P., DeLeo, J. A. & Romero-Sandoval, E. A. Mitogen activated protein kinase phosphatase-1 prevents the development of tactile sensitivity in a rodent model of neuropathic pain. Mol. Pain 8, 34 (2012).
Romero-Sandoval, E. A., Horvath, R., Landry, R. P. & DeLeo, J. A. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol. Pain 5, 25 (2009).
Turrin, N. P. & Rivest, S. Tumor necrosis factor-α but not interleukin-1β mediates neuroprotection in response to acute nitric oxide excitotoxicity. J. Neurosci. 26, 143–151 (2006).
Nadeau, S. et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J. Neurosci. 31, 12533–12542 (2011).
Fontaine, V. et al. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J. Neurosci. 22, RC216 (2002).
Varrassi, G. Management of chronic pain. Foreword. Clin. Drug Investig. 30 (Suppl. 2), 1 (2010).
Saito, O. et al. Spinal glial TLR4-mediated nociception and production of prostaglandin E2 and TNF. Br. J. Pharmacol. 160, 1754–1764 (2010).
Sweitzer, S. & De Leo, J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol. 2011, 235–250 (2011).
Rolan, P., Hutchinson, M. & Johnson, K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin. Pharmacother. 10, 2897–2904 (2009).
Nikodemova, M., Duncan, I. D. & Watters, J. J. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IκBα degradation in a stimulus-specific manner in microglia. J. Neurochem. 96, 314–323 (2006).
Sumracki, N. M. et al. The effects of pregabalin and the glial attenuator minocycline on the response to intradermal capsaicin in patients with unilateral sciatica. PLoS ONE 7, e38525 (2012).
Landry, R. P., Jacobs, V. L., Romero-Sandoval, E. A. & DeLeo, J. A. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp. Neurol. 234, 340–350 (2012).
Watkins, L. R. et al. Propentofylline, a CNS glial modulator, does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp. Neurol. 234, 351–353 (2012).
Plane, J. M., Shen, Y., Pleasure, D. E. & Deng, W. Prospects for minocycline neuroprotection. Arch. Neurol. 67, 1442–1448 (2010).
Imbesi, M., Uz, T., Manev, R., Sharma, R. P. & Manev, H. Minocycline increases phosphorylation and membrane insertion of neuronal GluR1 receptors. Neurosci. Lett. 447, 134–137 (2008).
Kobayashi, K. et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 4, e525 (2013).
Wang, X. et al. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 27, 2713–2722 (2013).
Lewis, S. S. et al. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J. Pain 13, 498–506 (2012).
Hutchinson, M. R. et al. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 7, 98–111 (2007). The first report demonstrating that opioids signal via TLR4.
Hutchinson, M. R. et al. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience 168, 551–563 (2010).
Nagata, K. et al. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol. Pain 5, 20 (2009).
Anand, P. et al. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain 15, 1040–1048 (2011).
Ji, R. R., Xu, Z. Z., Strichartz, G. & Serhan, C. N. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 34, 599–609 (2011).
Xu, Z. Z. et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nature Med. 16, 592–597 (2010).
Simonato, M. et al. Progress in gene therapy for neurological disorders. Nature Rev. Neurol. 9, 277–291 (2013).
Soderquist, R. G. et al. Release of plasmid DNA-encoding IL-10 from PLGA microparticles facilitates long-term reversal of neuropathic pain following a single intrathecal administration. Pharm. Res. 27, 841–854 (2010).
Shi, Y., Gelman, B. B., Lisinicchia, J. G. & Tang, S. J. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J. Neurosci. 32, 10833–10840 (2012).
Del Valle, L., Schwartzman, R. J. & Alexander, G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav. Immun. 23, 85–91 (2009).
Alexander, G. M., van Rijn, M. A., van Hilten, J. J., Perreault, M. J. & Schwartzman, R. J. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 116, 213–219 (2005).
Syberg, S. et al. Association between P2X7 receptor polymorphisms and bone status in mice. J. Osteoporos 2012, 637986 (2012).
Hutchinson, M. R. et al. Low-dose endotoxin potentiates capsaicin-induced pain in man: evidence for a pain neuroimmune connection. Brain Behav. Immun. 30, 3–11 (2013).
Kalliomaki, J. et al. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain 154, 761–767 (2013).
Banati, R. B. et al. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport 12, 3439–3442 (2001).
Trapp, B. D. & Nave, K. A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 (2008).
Robberecht, W. & Philips, T. The changing scene of amyotrophic lateral sclerosis. Nature Rev. Neurosci. 14, 248–264 (2013).
Grace, P. M., Watkins, L. R. & Hutchinson, M. R. in Pain Comorbidities: Understanding and Treating the Complex Patient 1st edn (eds Giamberardino, M. & Jensen, T.) 137–156 (IASP Press, 2012).
Mogil, J. S. Animal models of pain: progress and challenges. Nature Rev. Neurosci. 10, 283–294 (2009).
Bennett, G. J. & Xie, Y. K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988).
Seltzer, Z., Dubner, R. & Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218 (1990).
Kim, S. H. & Chung, J. M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 (1992).
Grace, P. M., Hutchinson, M. R., Manavis, J., Somogyi, A. A. & Rolan, P. E. A novel animal model of graded neuropathic pain: utility to investigate mechanisms of population heterogeneity. J. Neurosci. Methods 193, 47–53 (2010).
Polomano, R. C., Mannes, A. J., Clark, U. S. & Bennett, G. J. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94, 293–304 (2001).
Sloane, E. et al. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental multiple sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav. Immun. 23, 92–100 (2009).
King, T. et al. Unmasking the tonic-aversive state in neuropathic pain. Nature Neurosci. 12, 1364–1366 (2009).
Langford, D. J. et al. Coding of facial expressions of pain in the laboratory mouse. Nature Methods 7, 447–449 (2010).
Grace, P. M., Strand, K. A., Maier, S. F. & Watkins, L. R. Suppression of voluntary wheel running in rats is dependent on the site of inflammation: evidence for voluntary running as a measure of hindpaw-evoked pain. J. Pain. 15, 121–128 (2014).
Monassi, C. R., Bandler, R. & Keay, K. A. A subpopulation of rats show social and sleep-waking changes typical of chronic neuropathic pain following peripheral nerve injury. Eur. J. Neurosci. 17, 1907–1920 (2003).
Mogil, J. S., Davis, K. D. & Derbyshire, S. W. The necessity of animal models in pain research. Pain 151, 12–17 (2010).
Scott, F. T. et al. A study of shingles and the development of postherpetic neuralgia in East London. J. Med. Virol. 70, S24–S30 (2003).
Kleibeuker, W. et al. IL-1β signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav. Immun. 22, 200–208 (2008).
Kleibeuker, W. et al. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur. J. Neurosci. 25, 1696–1704 (2007).
Willemen, H. L. et al. Microglial/macrophage GRK2 determines duration of peripheral IL-1β-induced hyperalgesia: contribution of spinal cord CX3CR1, 38 and IL-1 signaling. Pain 150, 550–560 (2010).
Loram, L. C. et al. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav. Immun. 25, 1408–1415 (2011).
Hains, L. E. et al. Prior laparotomy or corticosterone potentiates lipopolysaccharide-induced fever and sickness behaviors. J. Neuroimmunol. 239, 53–60 (2011).
Hains, L. E. et al. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J. Pain 11, 1004–1014 (2010).
Loram, L. C. et al. Prior exposure to repeated morphine potentiates mechanical allodynia induced by peripheral inflammation and neuropathy. Brain Behav. Immun. 26, 1256–1264 (2012).
Hutchinson, M. R. et al. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol. Rev. 63, 772–810 (2011). A comprehensive review of opioid-induced central immune signalling.
Wang, X. et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl Acad. Sci. USA 109, 6325–6330 (2012).
Hutchinson, M. R. & Watkins, L. R. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 76 Pt B, 218–227 (2013).
Ferrini, F. et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nature Neurosci. 16, 183–192 (2013).
Acknowledgements
Funding sources: P.M.G. is a CJ Martin Fellow of the Australian Government National Health and Medical Research Council and an American Australian Association Sir Keith Murdoch Fellow. M.R.H. is an Australian Research Council Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Celsus
-
Aulus Cornelius Celsus was a first century Roman encyclopaedist who gathered extensive writings from the Greek empire and translated them into Latin. In his great work De Medicina, he characterized the four cardinal signs of inflammation: heat, pain, swelling and redness.
- Sickness response
-
A defence mechanism triggered by the recognition of anything foreign to the host. An organized constellation of responses initiated by the immune system but co-ordinated and partially created by the brain, including physiological responses (for example, fever, increased sleep, hyperalgesia and allodynia), behavioural responses (for example, decreased social interaction, sexual activity, and food and water intake), and hormonal responses (increased release of classic hypothalamo–pituitary–adrenal and sympathetic hormones).
- Hyperalgesia
-
Increased pain from a stimulus that normally provokes pain. A form of nociceptive hypersensitivity.
- Peripheral sensitization
-
Increased responsiveness and reduced threshold of nociceptive neurons in the periphery to the stimulation of their receptive fields, such as that developing upon inflammation.
- Glia
-
Resident non-neuronal cells in the nervous system first described by Rudolf Virchow in 1856, that maintain the structural integrity of the nervous system, provide trophic support to neurons, insulate one neuron from another, and destroy pathogens and clear debris. Astrocytes and microglia are among the most thoroughly characterized glial cells and are immunocompetent.
- Allodynia
-
Pain in response to a stimulus that does not normally provoke pain. A form of nociceptive hypersensitivity.
- Gliosis
-
The transition from a role in physiological maintenance or surveillance to a reactive phenotype that is characterized by changes in cell number, morphology, phenotype, motility, protein expression and by the release of immunoregulatory products.
- Neuroimmune interface
-
Proposed to describe the bidirectional, modulatory signalling between immune cells and neurons. We argue that such interfaces are formed in the central nervous system and can explain the sensory adaptations underlying pathological pain.
- Central immune signalling
-
The process and consequences of immune mediator release by reactive immunocompetent cells in the central nervous system.
- Nociceptive pain
-
Physiological pain produced by intense noxious stimuli that activate high-threshold nociceptor neurons.
- Inflammatory pain
-
Occurs in response to tissue injury and the subsequent inflammatory response.
- First-order primary afferent neurons
-
Sensory neurons the cell bodies of which lie in the trigeminal or spinal dorsal root ganglia, with projections that transmit nociceptive signals from the periphery (the peripheral terminal) to the spinal cord (the central terminal).
- Central synapses
-
The synapses between first-order neurons, which form the presynaptic terminal, and nociceptive projection neurons, which form the postsynaptic terminal.
- Second-order nociceptive projection neurons
-
Neurons projecting from the medullary dorsal horn or the superficial laminae of the spinal dorsal horn to the brainstem or thalamic nuclei.
- Spinal dorsal horn
-
Two longitudinal subdivisions of grey matter in the posterior part of the spinal cord that receive terminals from afferent fibres originating from each side of the body that encode several types of sensory information, including nociception.
- Hindbrain
-
Also known as the rhombencephalon. An area rostral to the spinal cord that gives rise to the cerebellum, pons and medulla.
- Nociception
-
The neural process of encoding noxious mechanical, thermal and/or chemical stimuli that occurs in afferent fibres, which send signals to the central nervous system. Pain is expressed as the complex emotional and behavioural response to the central integration of nociceptive signals.
- Third-order neurons
-
Sensory neuronal projections originating from thalamic nuclei.
- Central sensitization
-
A period of facilitated transmission in nociceptive projection neurons that is characterized by a decreased activation threshold and an increased responsiveness to nociceptive stimuli.
- Windup
-
Cumulative increases in membrane depolarization elicited by repeated C-fibre stimulation.
- Masseter
-
A facial muscle that has a major role in chewing.
- Blood–CNS barrier
-
A barrier formed by astrocyte end-feet and the tight junctions between endothelial cells lining blood vessels that excludes constituents of the systemic circulation from entry into the central nervous system (CNS). It forms an important boundary between the sensitive microenvironment of the CNS and the relatively volatile environment of the systemic circulation.
- Trigeminal sensory neurons
-
Neurons found in the trigeminal nerve that mediate facial sensation and motor functions, including biting and chewing.
- Dorsal root ganglia
-
(DRG). The cell bodies of sensory neurons are collected together in paired ganglia that lie alongside the spinal cord. Each cell body is encapsulated by satellite glia, with the entire ganglia being surrounded by a capsule of connective tissue and a perineurium.
- Brain-derived neurotrophic factor
-
(BDNF). A neurotrophin expressed at high levels in the central nervous system that is vital for the growth and survival of neurons, and has been implicated in many forms of synaptic plasticity.
- C-fibres
-
Small diameter, unmyelinated primary afferent sensory fibres, with small cell bodies in the dorsal root ganglion. Some C-fibres are mechanically insensitive, but most are polymodal, responding to noxious, thermal, mechanical and chemical stimuli.
- Lamina I neurons
-
The most superficial aspect of the spinal dorsal horn from which second-order nociceptive projection neurons originate.
- Reversal potential
-
The membrane potential at which chemical and electrical drive are equal and opposite, such that there is no net flow of ions across the membrane. The direction of flow reverses above and below this potential.
- Anti-allodynic and anti-hyperalgesic
-
Compounds that oppose allodynia and hyperalgesia to restore basal sensory thresholds.
- Radiculopathy
-
A condition arising from a compressed spinal nerve root that is associated with pain, numbness, tingling or weakness along the course of the nerve.
- Carpal tunnel syndrome
-
A syndrome arising from compression of the median nerve — which runs from the forearm into the palm of the hand — that is associated with itching numbness, burning and/or tingling.
- Complex regional pain syndrome
-
An idiopathic chronic pain condition that usually affects an arm or leg and typically develops after an injury, surgery, stroke or heart attack, but the pain is out of proportion to the severity of the initial injury, if any.
- Capsaicin
-
The active component of chilli peppers. It selectively binds to transient receptor potential cation channel subfamily V member 1 (TRPV1) on nociceptive and heat-sensing neurons.
- Postherpetic neuralgia
-
Neuropathic pain that occurs in some patients following infection with the varicella zoster virus, predominantly affecting the face.
- Peripheral benzodiazepine binding site
-
GABAA receptors in the central nervous system (CNS) represent the primary site of action for benzodiazepines, such as diazepam. The peripheral benzodiazepine binding site was originally discovered in the periphery a secondary binding site, but has since been identified in the CNS.
Rights and permissions
About this article
Cite this article
Grace, P., Hutchinson, M., Maier, S. et al. Pathological pain and the neuroimmune interface. Nat Rev Immunol 14, 217–231 (2014). https://doi.org/10.1038/nri3621
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3621
This article is cited by
-
NLRs and inflammasome signaling in opioid-induced hyperalgesia and tolerance
Inflammopharmacology (2024)
-
Identification and validation of biomarkers related to Th1 cell infiltration in neuropathic pain
Journal of Inflammation (2023)
-
Inducible co-stimulatory molecule (ICOS) alleviates paclitaxel-induced neuropathic pain via an IL-10-mediated mechanism in female mice
Journal of Neuroinflammation (2023)
-
Pain-resolving immune mechanisms in neuropathic pain
Nature Reviews Neurology (2023)
-
Post-injury pain and behaviour: a control theory perspective
Nature Reviews Neuroscience (2023)