Abstract
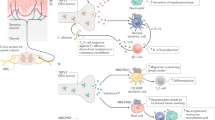
Pain involves neuroimmune crosstalk, but the mechanisms of this remain unclear. Here we showed that the splenic T helper 2 (TH2) immune cell response is differentially regulated in male mice with acute versus chronic neuropathic pain and that acetylcholinergic neurons in the dorsal motor nucleus of the vagus (AChDMV) directly innervate the spleen. Combined in vivo recording and immune cell profiling revealed the following two distinct circuits involved in pain-mediated peripheral TH2 immune response: glutamatergic neurons in the primary somatosensory cortex (GluS1HL)→AChDMV→spleen circuit and GABAergic neurons in the central nucleus of the amygdala (GABACeA)→AChDMV→spleen circuit. The acute pain condition elicits increased excitation from GluS1HL neurons to spleen-projecting AChDMV neurons and increased the proportion of splenic TH2 immune cells. The chronic pain condition increased inhibition from GABACeA neurons to spleen-projecting AChDMV neurons and decreased splenic TH2 immune cells. Our study thus demonstrates how the brain encodes pain-state-specific immune responses in the spleen.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (Z.Z.) upon request.
References
Gao, X. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021).
Kuner, R. & Kuner, T. Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev. 101, 213–258 (2021).
Grace, P. M., Hutchinson, M. R., Maier, S. F. & Watkins, L. R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 14, 217–231 (2014).
Baral, P., Udit, S. & Chiu, I. M. Pain and immunity: implications for host defence. Nat. Rev. Immunol. 19, 433–447 (2019).
Ji, R. R., Chamessian, A. & Zhang, Y. Q. Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 (2016).
Mohanta, S. K. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022).
Chiu, I. M., von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
Liu, S. et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 598, 641–645 (2021).
Tanaka, S. et al. Vagus nerve stimulation activates two distinct neuroimmune circuits converging in the spleen to protect mice from kidney injury. Proc. Natl Acad. Sci. USA 118, e2021758118 (2021).
Xu, Y. et al. Pituitary hormone α-MSH promotes tumor-induced myelopoiesis and immunosuppression. Science 377, 1085–1091 (2022).
Bassi, G. S. et al. Anatomical and clinical implications of vagal modulation of the spleen. Neurosci. Biobehav. Rev. 112, 363–373 (2020).
Liu, D. et al. CD97 promotes spleen dendritic cell homeostasis through the mechanosensing of red blood cells. Science 375, eabi5965 (2022).
Verlinden, T. J. M. et al. Innervation of the human spleen: a complete hilum-embedding approach. Brain Behav. Immun. 77, 92–100 (2019).
Schiller, M., Ben-Shaanan, T. L. & Rolls, A. Neuronal regulation of immunity: why, how and where? Nat. Rev. Immunol. 21, 20–36 (2021).
Veerakumar, A., Yung, A. R., Liu, Y. & Krasnow, M. A. Molecularly defined circuits for cardiovascular and cardiopulmonary control. Nature 606, 739–746 (2022).
Kaelberer, M. M., Rupprecht, L. E., Liu, W. W., Weng, P. & Bohorquez, D. V. Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu. Rev. Neurosci. 43, 337–353 (2020).
Pavlov, V. A. & Tracey, K. J. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 20, 156–166 (2017).
Okusa, M. D., Rosin, D. L. & Tracey, K. J. Targeting neural reflex circuits in immunity to treat kidney disease. Nat. Rev. Nephrol. 13, 669–680 (2017).
Winkelmann, A., Loebermann, M., Barnett, M., Hartung, H. P. & Zettl, U. K. Vaccination and immunotherapies in neuroimmunological diseases. Nat. Rev. Neurol. 18, 289–306 (2022).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Hu, B. et al. γδT cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020).
Kaelberer, M. M. et al. A gut–brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Kressel, A. M. et al. Identification of a brainstem locus that inhibits tumor necrosis factor. Proc. Natl Acad. Sci. USA 117, 29803–29810 (2020).
Cailotto, C. et al. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 24, 191–200 (2012).
Chavan, S. S., Pavlov, V. A. & Tracey, K. J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46, 927–942 (2017).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020).
Faust, H. J. et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Invest. 130, 5493–5507 (2020).
Milligan, E. D. & Watkins, L. R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 (2009).
Gangadharan, V. et al. Neuropathic pain caused by miswiring and abnormal end organ targeting. Nature 606, 137–145 (2022).
Bethea, J. R. & Fischer, R. Role of peripheral immune cells for development and recovery of chronic pain. Front. Immunol. 12, 641588 (2021).
Kavelaars, A. & Heijnen, C. J. Immune regulation of pain: friend and foe. Sci. Transl. Med. 13, eabj7152 (2021).
Costigan, M. et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 29, 14415–14422 (2009).
Mota, C. M. D. & Madden, C. J. Neural control of the spleen as an effector of immune responses to inflammation: mechanisms and treatments. Am. J. Physiol. Regul. Integr. Comp. Physiol. 323, R375–R384 (2022).
Susaki, E. A. et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 10, 1709–1727 (2015).
Xiao, X. et al. A genetically defined compartmentalized striatal direct pathway for negative reinforcement. Cell 183, 211–227 (2020).
Ren, J. et al. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175, 472–487 (2018).
Zhu, X. et al. Distinct thalamocortical circuits underlie allodynia induced by tissue injury and by depression-like states. Nat. Neurosci. 24, 542–553 (2021).
Zhuo, M. Cortical excitation and chronic pain. Trends Neurosci. 31, 199–207 (2008).
Zhou, W. et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat. Neurosci. 22, 1649–1658 (2019).
Zhu, X. et al. A central amygdala input to the parafascicular nucleus controls comorbid pain in depression. Cell Rep. 29, 3847–3858.e5 (2019).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Travagli, R. A., Hermann, G. E., Browning, K. N. & Rogers, R. C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 68, 279–305 (2006).
Reilly, F. D., McCuskey, R. S. & Meineke, H. A. Studies of the hemopoietic microenvironment. VIII. Andrenergic and cholinergic innervation of the murine spleen. Anat. Rec. 185, 109–117 (1976).
Nance, D. M. & Burns, J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav. Immun. 3, 281–290 (1989).
Bellinger, D. L., Lorton, D., Hamill, R. W., Felten, S. Y. & Felten, D. L. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 7, 191–204 (1993).
Cano, G., Sved, A. F., Rinaman, L., Rabin, B. S. & Card, J. P. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 439, 1–18 (2001).
Gautron, L. et al. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J. Comp. Neurol. 521, 3741–3767 (2013).
Buijs, R. M., van der Vliet, J., Garidou, M. L., Huitinga, I. & Escobar, C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS ONE 3, e3152 (2008).
Kudoh, G., Hoshi, K. & Murakami, T. Fluorescence microscopic and enzyme histochemical studies of the innervation of the human spleen. Arch. Histol. Jpn. 42, 169–180 (1979).
Basbaum, A. I., Bautista, D. M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Koren, T. et al. Insular cortex neurons encode and retrieve specific immune responses.Cell 184, 5902–5915.e17 (2021).
Fan, K. Q. et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179, 864–879 (2019).
Qing, H. et al. Origin and function of stress-induced IL-6 in murine models. Cell 182, 372–387 (2020).
Ben-Shaanan, T. L. et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 9, 2723 (2018).
Zhang, Z., Li, Y., Lv, X., Zhao, L. & Wang, X. VLM catecholaminergic neurons control tumor growth by regulating CD8+ T cells. Proc. Natl Acad. Sci. USA 118, e2103505118 (2021).
McMahon, S. B., La Russa, F. & Bennett, D. L. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci. 16, 389–402 (2015).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Filiano, A. J., Gadani, S. P. & Kipnis, J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci. 18, 375–384 (2017).
Shin, G. et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521 (2017).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Gong, R., Xu, S., Hermundstad, A., Yu, Y. & Sternson, S. M. Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell 182, 1589–1605 (2020).
Liu, Y. et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018).
Sheintuch, L. et al. Tracking the same neurons across multiple days in Ca2+ imaging data. Cell Rep. 21, 1102–1115 (2017).
Chen, Y. et al. A versatile tiling light sheet microscope for imaging of cleared tissues. Cell Rep. 33, 108349 (2020).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (STI2030-Major Projects 2021ZD0203100), the National Natural Science Foundation of China (grants 32025017, 32241013, 32100808 and 32121002), the Plans for Major Provincial Science & Technology Projects (202303a07020002), CAS Project for Young Scientists in Basic Research (YSBR-013), China National Postdoctoral Program for Innovative Talents (BX2021286), the China Postdoctoral Science Foundation (2020M682018), the Fundamental Research Funds for the Central Universities (WK9100000005), the Institute of Health and Medicine (QYZD20220007) and also supported by the Innovative Research Team of High-level Local Universities in Shanghai. We would like to acknowledge the Confocal Imaging Unit at the Core Facility Center for Life Science of USTC. We would like to thank Z. Liu (University of Science and Technology of China) for valuable technical expertise and assistance.
Author information
Authors and Affiliations
Contributions
X.Z., J.Y.-H., W.Y.-D. and H.D.-T. designed the studies, conducted most of the experiments and data analysis, and wrote the draft manuscript. Q.W. and H.Z. conducted some of the molecular and behavioral experiments. P.K.-C., Y.J., M.Y.-Z., W.Z. and Y.M. conducted the behavioral experiments and data analyses and wrote the text of the final manuscript. Haitao Wang and Y.Z. were involved in the overall design of the study. S.X., Y.T., Hao Wang and W.T. were involved in the revision of the manuscript. L.B. and Z.Z. were involved in the overall design of the project, individual experiments, data analysis and the writing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Rohini Kuner, Kevin Tracey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Neuropathic pain-induced changes in splenic immune cell populations.
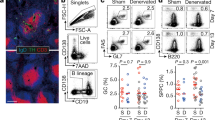
a, Gating strategy of the different immune cell subpopulations in the splenic immune cells analyzed by flow cytometry. b–d Flow cytometry dot plots (left) and summary data (right) of B cells (b), T cells (c), and NK cells (d) in the spleen of 1D, 1W, 2W, 3W, and 4W SNI mice (and time-matched sham mice). e, Flow cytometry dot plots (left) and summary data (right) of CD8 T cells in the spleen of 1D, 1W, 2W, 3W, and 4W SNI model mice (and time-matched sham mice). f, Flow cytometry dot plots (left) and summary data (right) of CD4 T cells in the spleen of 1D (t24 = 2.167, P = 0.0404), 1W (t19 = 0.4459, P = 0.6607), 2W (t11 = 1.643, P = 0.1287), 3W (t10 = 0.4361, P = 0.6721) and 4W (t23 = 3.878, P < 0.001) SNI mice (and time-matched sham mice). Significance was assessed by two-tailed unpaired Student’s t-test in (b–f). All data are presented as the mean ± SEM. *P < 0.05, ***P < 0.001, not significant (n.s.). Details of the statistical analyses are presented in Supplementary Table 1.
Extended Data Fig. 2 Effects of morphine treatment on pain threshold and on the proportion of splenic TH2 immune cells in SNI mice.
a, Schematic for the morphine treatment of SNI 1D mice. b,c, Effects on pain threshold (b) and the proportion of CD4+ IL-4+ and CD4+ IL-10+ cells in spleens (c) of SNI 1D mice after morphine treatment. d, Schematic for morphine treatment of SNI 4W mice. e,f, Effects on pain threshold (e) and the proportion of CD4+ IL-4+ and CD4+ IL-10+ cells in spleens (f) of SNI 4W mice after morphine treatment. Significance was assessed by two-way repeated-measures ANOVA with post hoc comparison between groups in (b,e), and two-tailed unpaired Student’s t-test in (c,f). All data are presented as the mean ± SEM. *P < 0.05, ***P < 0.001. Details of the statistical analyses are presented in Supplementary Table 1.
Extended Data Fig. 3 The FG signal in the splenic nerve and vagal efferent following FG injection into the spleen.
a, Schematic diagram for Flouro-Gold (FG) injection into the spleen of C57 mice. b,c, Representative images showing FG co-localization with TUBB3 antibody in the splenic nerve (b) and vagus nerve (c). Scale bars, 50 µm. d, Representative images showing the FG signals in the celiac ganglion. Scale bar, 10 µm.
Extended Data Fig. 4 Mapping inputs of the spleen in the whole brain.
a, Schematic diagram for Flouro-Gold (FG) injection into spleens of C57 mice. b, A series of coronal sections, from a representative mouse, showing the distributions of FG signals across different brain areas. Scale bars, 500 μm or 50 μm (zoom). c, Graphs showing the fraction of FG labeled neurons in each brain region that projected to the spleen (n = 5 slice from 3 mice). S1, primary somatosensory cortex; S2, secondary somatosensory cortex; BST, bed nucleus of the stria terminalis; AID, agranular insular cortex, dorsal part; AIV, agranular insular cortex, ventral part; AIP, agranular insular cortex, posterior part; Cl, claustrum; DEn, dorsal endopiriform nucleus; Pir, piriform cortex; S1FL, primary somatosensory cortex, forelimb region; S1BF, primary somatosensory cortex, barrel field; LGP, lateral geniculate nucleus; VP, ventral pallidum; IC, insular cortex; S1DZ, primary somatosensory cortex, dysgranular region; VPM, ventral posteromedial thalamic nucleus; VM, ventromedial thalamic nucleus; ic, internal capsule; MGP, medial globus pallidus; LH, lateral hypothalamic area; ZI, zona incerta; La, lateral amygdaloid nucleus; BLA, basolateral amygdaloid nucleus; LPtA, lateral parietal association cortex; V2, secondary visual cortex; AuD, secondary auditory cortex, dorsal area; cp, cerebral peduncle; STh, subthalamic nucleus; BMP, basomedial amygdaloid nucleus; SC, suprachiasmatic nucleus; V1, primary visual cortex; VTA, ventral tegmental area; SNR, substantia nigra; Ect, ectorhinal cortex; PRh, perirhinal cortex; LEnt, lateral entorhinal cortex; PAG, periaqueductal gray; ECIC, external cortex of the inferior colliculus; PnO, pontine reticular nucleus, oral part; Pr5, principal sensory trigeminal nucleus; 3Cb, 3rd Cerebellar lobule; Sim, simple lobule; PDTg, posterodorsal tegmental nucleus; 6n, root of abducens nerve; 7n, facial nerve or its root; 8vn,vestibular root of the vestibulocochlear nerve; DMV, dorsal motor nucleus of the vagus; NA, ambiguous nucleus; 12N, hypoglossal nucleus. All data are presented as the mean ± SEM.
Extended Data Fig. 5 FG signal in the DMV upon FG injection into the spleen following neurectomy.
a, Schematic diagram for Flouro-Gold (FG) injection into the spleen of C57 mice. b, Representative images showing the FG signal in the DMV, at 7 days after spleen injection of FG. Scale bar, 100 µm. c, Schematic diagram for FG injection into the spleen of C57 mice after celiac ganglion transection. d, Representative images showing the FG signal in the DMV, at 7 days after spleen injection of FG and celiac ganglion transection. Scale bar, 100 µm. e, Schematic diagram for FG injection into the spleen of C57 mice after splenic nerve denervation. f, Representative images showing the FG signal in the DMV, at 7 days after spleen injection of FG and splenic nerve denervation. Scale bar, 100 µm.
Extended Data Fig. 6 Triple retrograde tracing of GluS1HL→AChDMV→spleen and GABACeA→AChDMV→spleen circuits.
a, Schematic diagram for the tracing strategy using a Cre-dependent retrograde trans-monosynaptic rabies virus. b, Representative confocal image of the starter neurons (yellow) within the DMV. Scale bar, 50 μm. The smaller images on the right depict the area shown in the box of the DMV. Scale bar, 10 μm. c, Representative confocal image of RV-DsRed expression in the BNST and PVN. Scale bars, 100 μm. d, Left: representative confocal image of RV-DsRed expression in the S1HL. Scale bar, 100 µm. Right: DsRed-labeled neurons in the S1HL co-localized with glutamatergic immunofluorescence. Scale bar, 10 μm. e, Left: representative confocal image of RV-DsRed expression in the CeA. Scale bar, 100 µm. Right: DsRed-labeled neurons in the CeA co-localized with GABA immunofluorescence. Scale bar, 10 μm. f, Quantitative analysis showing that 81% of S1HL-projecting neurons co-localized with ChAT antibody in the DMV (n = 5 slice from 3 mice). g, Quantitative analysis showing that 79% of CeA-projecting neurons co-localized with ChAT antibody in the DMV (n = 5 slice from 3 mice). All data are presented as the mean ± SEM.
Extended Data Fig. 7 Mapping outputs of the GluS1HL and GABACeA neurons.
a, Schematic diagram of AAV-DIO-ChR2-EYFP injected into the S1HL of CaMK2-Cre mice. b, A representative confocal image of AAV-DIO-ChR2-EYFP expression in the S1HL. Scale bar, 100 µm. c, Representative confocal images of EYFP+ fiber expression in the CPu, S2, cp, PO, VPM and Cu of mice injected with AAV-DIO-ChR2-EYFP into the S1HL. Scale bar, 100 µm. d, Schematic diagram of AAV-DIO-ChR2-EYFP injected into the CeA of GAD2-Cre mice. e, Representative confocal image of AAV-DIO-ChR2-EYFP expression in the CeA. Scale bar, 100 µm. f, Representative confocal images of EYFP+ fiber expression in the BNST, PVN, VMH, LHb, PF and PAG of mice injected with AAV-DIO-ChR2-EYFP into the CeA. Scale bar, 100 µm. CPu, caudate putamen; S2, secondary somatosensory cortex; cp, cerebral peduncle; PO, posterior thalamic nuclear; VPM, ventral posteromedial thalamic nucleus; Cu, cuneate nucleus; BNST, bed nucleus of the stria terminalis; PVN, paraventricular thalamic nucleus; VMH, ventromedial hypothalamic nucleus; LHb, lateral habenular nucleus; PF, parafascicular thalamic nucleus; PAG, periaqueductal gray.
Extended Data Fig. 8 c-Fos expression in the S1HL and CeA from SNI mice.
a,b, Representative confocal images (a) and quantitative analysis (b, SNI 1D/Sham 1D: S1HL vs CeA; F1,10 = 1434, left, P < 0.001; right, P = 0.05150) showing c-Fos+ and EYFP+ neurons in the S1HL and CeA of sham 1D and SNI 1D mice. Scale bar, 100 µm. c,d, Representative confocal images (c) and quantitative analysis (d, SNI 4W/Sham 4W: S1HL vs CeA; F1,10 = 638.5, left, P = 0.2245; right, P < 0.001) showing c-Fos+ and EYFP+ neurons in the S1HL and CeA of sham 4W and SNI 4W mice. Scale bar, 100 µm. Significance was assessed by two-way repeated-measures ANOVA with post hoc comparison between groups (b,d). All data are presented as the mean ± SEM. ***P < 0.001, not significant (n.s.). Details of the statistical analyses are presented in Supplementary Table 1.
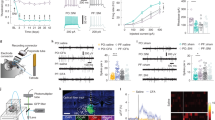
Extended Data Fig. 9 GluS1HL neuronal activity is enhanced in SNI 1D mice.
a, Schematic of retro-AAV-hSyn-Cre injection in the DMV and recording configuration in the S1HL acute slices. b, A representative image of DMV-projecting GluS1HL neurons labeled by retro-AAV-hSyn-Cre injected into the DMV of C57 mice. Scale bar, 100 µm. c,d, Sample traces and statistical data for firing rate (c) and rheobase (d; t28 = 3.974, P < 0.001) recorded from DMV-projecting GluS1HL neurons in sham 1D or SNI 1D mice. e,f, Sample traces and statistical data for firing rate (e) and rheobase (f) recorded from DMV-projecting GluS1HL neurons in sham 4W or SNI 4W mice. Significance was assessed by two-way repeated-measures ANOVA with post hoc comparison between groups (c,e), and two-tailed paired Student’s t-test in d,f. All data are presented as the mean ± SEM. **P < 0.01, ***P < 0.001, not significant (n.s.). Details of the statistical analyses are presented in Supplementary Table 1.
Extended Data Fig. 10 GABACeA neuronal activity is enhanced in SNI 4W mice.
a, Schematic of retro-AAV-hSyn-Cre injection in the DMV and recording configuration in the CeA acute slices. b, A representative image of DMV-projecting GABACeA neurons labeled by retro-AAV-hSyn-Cre injected into the DMV of C57 mice. Scale bar, 100 µm. c,d, Sample traces and statistical data for firing rate (c) and rheobase (d) recorded from DMV-projecting GABACeA neurons in sham 1D or SNI 1D mice. e,f, Sample traces and statistical data for firing rate (e) and rheobase (f) recorded from DMV-projecting GABACeA neurons in sham 4W or SNI 4W mice. g, Schematic for injection of AAV-DLX5/6-eNpHR-EYFP into the CeA of C57 mice and of AAV-ChAT-hM4Di-mCherry into the DMV; optical fibers were implanted above the DMV, cannulas were implanted above the NTS. h, Effects on the percentages of CD4+ IL-4+ and CD4+ IL-10+ cells in the spleen of SNI 4W mice after optogenetic inhibition (594 nm, constant) the CeA→DMV pathway and chemogenetic inhibition of the DMV→NTS pathway. Significance was assessed by two-way repeated-measures ANOVA with post hoc comparison between groups (c,e), and two-tailed unpaired Student’s t-test in d,f,h. All data are presented as the mean ± SEM. *P < 0.05, not significant (n.s.). Details of the statistical analyses are presented in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Note (detailed methods), Figs. 1–11 and Table 1.
Supplementary Video 1
Reconstruction image of FG and tdTomato signal in the DMV with FG injected into the spleen of ChAT-tdT mice. Scale bar, 100 μm.
Supplementary Video 2
In vivo microendoscopic calcium imaging in the DMV with spleen infusion of retro-AAV-hSyn-Cre and DMV infusion of AAV-DIO-GCaMP6f of C57 mice.
Supplementary Video 3
Reconstruction image of RV-DsRed expression in the CeA with retro-AAV-hSyn-Cre injected into the spleen and Cre-dependent helper viruses injected into the DMV of C57 mice. Scale bar, 100 μm.
Supplementary Video 4
In vivo two-photon calcium imaging in the S1HL with DMV infusion of retro-AAV-hSyn-Cre and S1HL infusion of AAV-DIO-GCaMP6f of C57 mice. Scale bar, 50 μm.
Supplementary Video 5
Optical-fiber-based calcium signals recording of DMV-projecting GluS1HL neurons evoked by 0.07 g von Frey filament stimuli on the injured paws of sham 1-d (left) and SNI 1-d (right) mice.
Supplementary Video 6
Optical-fiber-based calcium signals recording of DMV-projecting GluS1HL neurons evoked by 0.07 g von Frey filament stimuli on the injured paws of sham 4-week (left) and SNI 4-week (right) mice.
Supplementary Video 7
In vivo microendoscopic calcium imaging in the CeA with DMV infusion of retro-AAV-hSyn-Cre and CeA infusion of AAV-DIO-GCaMP6f of C57 mice.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, X., Huang, JY., Dong, WY. et al. Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen. Nat Neurosci 27, 471–483 (2024). https://doi.org/10.1038/s41593-023-01561-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01561-8