Key Points
-
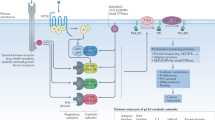
Sphingosine-1-phosphate (S1P) is a bioactive signalling molecule that is involved in several pathways that are crucial for health, and it has been implicated in disorders including cancer and inflammatory diseases. Efforts are underway to pharmacologically regulate S1P synthesis, degradation, export and receptor activation.
-
S1P is generated by two intracellular kinases — sphingosine kinase 1 (SPHK1) and SPHK2 — that are conserved from yeast to humans. SPHK1 typically promotes cell growth and inhibits apoptosis, and its upregulation has been linked to several types of cancer.
-
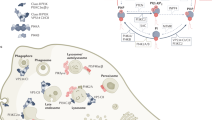
Intracellular S1P can be exported outside cells by several ATP-binding cassette (ABC) transporters and the major facilitator superfamily member SPNS2, which regulates S1P secretion from endothelial and lymphendothelial cells and is an attractive new therapeutic target. Many receptor agonists and stimuli induce the synthesis and export of S1P.
-
Extracellular S1P can bind to a family of five G protein-coupled receptors (S1P receptor 1 (S1PR1) to S1PR5) that can be coupled to multiple Gα proteins, thus leading to multiple cellular effects. These receptors are the targets of various compounds that are in different stages of drug development. In addition, S1PR1 is a target of fingolimod, which is used clinically for the treatment of multiple sclerosis.
-
S1PRs control the movement of cells into and out of the blood, circulating lymph and other tissues. Fingolimod and other compounds that target S1PR1 are being used to disrupt lymphocyte egress from lymph nodes in relapsing and remitting multiple sclerosis. Clinical trials are underway to investigate the efficacy of these drugs in other autoimmune diseases, including rheumatoid arthritis and lupus.
-
Other S1PRs are not as well studied, but they all have potential pathophysiological roles. S1PR2 typically inhibits the migration of osteoclasts and promotes osteoclast retention in bone and thus bone resorption. S1PR3 promotes cancer growth and vascular leakage during sepsis. S1PR4 has been implicated in certain immune cell functions, including dendritic cell function and T helper 17 (TH17) cell differentiation. Similar to the role of S1PR1 in lymphocyte trafficking, S1PR5 is required for the trafficking of natural killer cells into peripheral tissues.
Abstract
The bioactive lipid sphingosine-1-phosphate (S1P) is involved in multiple cellular signalling systems and has a pivotal role in the control of immune cell trafficking. As such, S1P has been implicated in disorders such as cancer and inflammatory diseases. This Review discusses the ways in which S1P might be therapeutically targeted — for example, via the development of chemical inhibitors that target the generation, transport and degradation of S1P and via the development of specific S1P receptor agonists. We also highlight recent conflicting results observed in preclinical studies targeting S1P and discuss ongoing clinical trials in this field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang, H. et al. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 114, 155–167 (1991). This paper provided the first evidence to demonstrate that S1P is a bioactive lipid produced from sphingosine that regulates cellular proliferation.
Fyrst, H. & Saba, J. D. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nature Chem. Biol. 6, 489–497 (2010).
Pyne, S. & Pyne, N. J. Translational aspects of sphingosine 1-phosphate biology. Trends Mol. Med. 17, 463–472 (2011).
Orr Gandy, K. A. & Obeid, L. M. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim. Biophys. Acta 1831, 157–166 (2013).
Maceyka, M., Harikumar, K. B., Milstien, S. & Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 (2012).
Rosen, H., Stevens, R. C., Hanson, M., Roberts, E. & Oldstone, M. B. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu. Rev. Biochem. 82, 637–662 (2013).
Pyne, N. J. & Pyne, S. Sphingosine 1-phosphate and cancer. Nature Rev. Cancer 10, 489–503 (2010).
Spiegel, S. & Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature Rev. Immunol. 11, 403–415 (2011).
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012).
Cuvillier, O. et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803 (1996). This paper established the concept of the sphingolipid rheostat complex, in which the balance between ceramide (the pro-apoptotic precursor of S1P) and the anti-apoptotic S1P determines cell fate.
Brinkmann, V. et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Rev. Drug Discov. 9, 883–897 (2010).
Alvarez, S. E. et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 (2010).
Hait, N. C. et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 (2009). This was the first demonstration that HDACs are direct intracellular targets of S1P and are formed in the nucleus by SPHK2, thus linking nuclear sphingolipid metabolism to gene regulation.
Takasugi, N. et al. BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. J. Neurosci. 31, 6850–6857 (2011).
Kim, R. H., Takabe, K., Milstien, S. & Spiegel, S. Export and functions of sphingosine-1-phosphate. Biochim. Biophys. Acta 1791, 692–696 (2009).
Kawahara, A. et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 (2009). This study showed that SPNS2 is an S1P transporter that has an important role in cardiac development in zebrafish. This study led to the later demonstration that mammalian SPNS2 analogues are also S1P transporters.
Nagahashi, M. et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 27, 1001–1011 (2013).
Hisano, Y., Kobayashi, N., Yamaguchi, A. & Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE 7, e38941 (2012).
Fukuhara, S. et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426 (2012).
Mendoza, A. et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2, 1104–1110 (2012).
Breart, B. et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208, 1267–1278 (2011).
Liang, J. et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 (2013).
Mizugishi, K. et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25, 11113–11121 (2005).
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Rev. Mol. Cell. Biol. 9, 139–150 (2008).
Lee, H. et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nature Med. 16, 1421–1428 (2010). This study showed that persistent activation of STAT3 in tumours involves S1P and S1PR1 in a malicious feedforward cycle.
Liu, Y. et al. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood 120, 1458–1465 (2012).
Deng, J. et al. S1PR1–STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell 21, 642–654 (2012).
Nagahashi, M. et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by tumor-induced angiogenesis and lymphangiogenesis. Cancer Res. 72, 726–735 (2012).
Ponnusamy, S. et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol. Med. 4, 761–775 (2012). This paper highlighted that S1P is involved in the communication between tumours and hosts to regulate metastasis.
Ruckhaberle, E. et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res. Treat. 112, 41–52 (2008).
Kawamori, T. et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 23, 405–414 (2008).
Pyne, S., Edwards, J., Ohotski, J. & Pyne, N. J. Sphingosine 1-phosphate receptors and sphingosine kinase 1: novel biomarkers for clinical prognosis in breast, prostate, and hematological cancers. Front. Oncol. 2, 168 (2012).
Paugh, S. W. et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 112, 1382–1391 (2008).
Kharel, Y. et al. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem. J. 440, 345–353 (2011).
Schnute, M. E. et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 444, 79–88 (2012).
Pyne, S., Bittman, R. & Pyne, N. J. Sphingosine kinase inhibitors and cancer: seeking the golden sword of Hercules. Cancer Res. 71, 6576–6582 (2011).
Wang, Z. et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure 21, 798–809 (2013).
Chipuk, J. E. et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148, 988–1000 (2012).
Gomez, L. et al. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Bas. Res. Cardiol. 106, 1341–1353 (2011).
Vessey, D. A. et al. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid. Med. Cell. Longev. 2011, 961059 (2011).
Strub, G. M. et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 25, 600–612 (2011).
French, K. J. et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 333, 129–139 (2010).
Antoon, J. W. et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology 151, 5124–5135 (2010).
Kharel, Y. et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem. J. 447, 149–157 (2012).
Samy, E. T. et al. Cutting edge: modulation of intestinal autoimmunity and IL-2 signaling by sphingosine kinase 2 independent of sphingosine 1-phosphate. J. Immunol. 179, 5644–5648 (2007).
Maines, L. W., Fitzpatrick, L. R., Green, C. L., Zhuang, Y. & Smith, C. D. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn's disease. Inflammopharmacology 18, 73–85 (2010).
Chumanevich, A. A. et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 31, 1787–1793 (2010).
Baker, D. A., Barth, J., Chang, R., Obeid, L. M. & Gilkeson, G. S. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J. Immunol. 185, 2570–2579 (2010).
Fitzpatrick, L. R. et al. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology 19, 75–87 (2011).
Kazantsev, A. G. & Thompson, L. M. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nature Rev. Drug Discov. 7, 854–868 (2008).
Haberland, M., Montgomery, R. L. & Olson, E. N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature Rev. Genet. 10, 32–42 (2009).
Fischer, A., Sananbenesi, F., Mungenast, A. & Tsai, L. H. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol. Sci. 31, 605–617 (2010).
Nakahara, K. et al. The Sjogren–Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol. Cell 46, 461–471 (2012). This study showed that the S1P metabolite hexadecenal is implicated in the pathogenesis of Sjögren–Larsson syndrome as a result of mutations in ALDH3A2 , which is needed to metabolize the hexedecanal produced from S1P by S1P lyase.
Aguilar, A. & Saba, J. D. Truth and consequences of sphingosine-1-phosphate lyase. Adv. Biol. Regul. 52, 17–30 (2012).
Ihlefeld, K., Claas, R. F., Koch, A., Pfeilschifter, J. M. & Meyer Zu Heringdorf, D. Evidence for a link between histone deacetylation and Ca2+ homoeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts. Biochem. J. 447, 457–464 (2012).
Schwab, S. R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005). This important study established that lymphocyte egress is mediated by S1P gradients that are established by S1P lyase activity.
Bagdanoff, J. T. et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). J. Med. Chem. 53, 8650–8662 (2010).
Bourquin, F., Riezman, H., Capitani, G. & Grutter, M. G. Structure and function of sphingosine-1-phosphate lyase, a key enzyme of sphingolipid metabolism. Structure 18, 1054–1065 (2010).
Nagata, Y., Partridge, T. A., Matsuda, R. & Zammit, P. S. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J. Cell Biol. 174, 245–253 (2006).
Loh, K. C. et al. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE 7, e37218 (2012).
Pantoja, M., Fischer, K. A., Ieronimakis, N., Reyes, M. & Ruohola-Baker, H. Genetic elevation of sphingosine 1-phosphate suppresses dystrophic muscle phenotypes in Drosophila. Development 140, 136–146 (2013).
Hanson, M. A. et al. Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 (2012). The first X-ray crystal structure of an S1PR, namely S1PR1, was reported in this paper. This structure provided a detailed view of the binding site of S1P and paves the way for the development of more specific agonists and antagonists that could be clinically useful.
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002). This study elucidated the mechanism of immunosuppression induced by fingolimod and showed that its phosphorylated form signals through S1PR1 to interfere with lymphocyte trafficking.
Brinkmann, V. et al. The immune modulator, FTY720, targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277, 21453–21457 (2002).
Graler, M. H. & Goetzl, E. J. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J. 18, 551–553 (2004).
Gonzalez-Cabrera, P. J. et al. S1P1 receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol. Pharmacol. 81, 166–174 (2012).
Neviani, P. et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J. Clin. Invest. 117, 2408–2421 (2007).
Gergely, P. et al. The selective S1P receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate: translation from preclinical to clinical studies. Br. J. Pharmacol. 167, 1035–1047 (2012).
Komiya, T. et al. Efficacy and immunomodulatory actions of ONO-4641, a novel selective agonist for sphingosine 1-phosphate receptors 1 and 5, in preclinical models of multiple sclerosis. Clin. Exp. Immunol. 171, 54–62 (2013).
Piali, L. et al. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J. Pharmacol. Exp. Ther. 337, 547–556 (2011).
Willis, M. A. & Cohen, J. A. Fingolimod therapy for multiple sclerosis. Semin. Neurol. 33, 37–44 (2013).
Nolan, R., Gelfand, J. M. & Green, A. J. Fingolimod treatment in multiple sclerosis leads to increased macular volume. Neurology 80, 139–144 (2013).
Quancard, J. et al. A potent and selective S1P1 antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem. Biol. 19, 1142–1151 (2012).
Choi, J. W. et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl Acad. Sci. USA 108, 751–756 (2011).
Sanna, M. G. et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chem. Biol. 2, 434–441 (2006).
Teijaro, J. R. et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146, 980–991 (2011). This study showed that S1PR1 agonists can suppress cytokines and innate immune cell recruitment, and it identified endothelial cells as central regulators of the cytokine storm induced by viral infections.
Matsuoka, Y., Nagahara, Y., Ikekita, M. & Shinomiya, T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br. J. Pharmacol. 138, 1303–1312 (2003).
Liu, Q. et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood 111, 275–284 (2008).
Roberts, K. G. et al. Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res. 70, 5438–5447 (2010).
Wang, Z., Jiang, H., Chen, S., Du, F. & Wang, X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148, 228–243 (2012).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Yu, H., Kortylewski, M. & Pardoll, D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Rev. Immunol. 7, 41–51 (2007).
Ding, B. B. et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 111, 1515–1523 (2008).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Rev. Cancer 9, 798–809 (2009).
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000). This is the first demonstration of the essential role of S1PR1 in vascular integrity.
Shoham, A. B. et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development 139, 3859–3869 (2012).
Gaengel, K. et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599 (2012).
Jung, B. et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 (2012).
Ishii, M. et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458, 524–528 (2009).
Ishii, M., Kikuta, J., Shimazu, Y., Meier-Schellersheim, M. & Germain, R. N. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 207, 2793–2798 (2010).
Kikuta, J. et al. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc. Natl Acad. Sci. USA 110, 7009–7013 (2013).
Long, J. S. et al. Sphingosine 1-phosphate receptor 4 uses HER2 (ERBB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J. Biol. Chem. 285, 35957–35966 (2010).
Salomone, S. & Waeber, C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front. Pharmacol. 2, 9 (2011).
Sukocheva, O. et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J. Cell Biol. 173, 301–310 (2006).
Watson, C. et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am. J. Pathol. 177, 2205–2215 (2010).
Harris, G. L., Creason, M. B., Brulte, G. B. & Herr, D. R. In vitro and in vivo antagonism of a G protein-coupled receptor (S1P3) with a novel blocking monoclonal antibody. PLoS ONE 7, e35129 (2012).
Sammani, S. et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am. J. Respir. Cell. Mol. Biol. 43, 394–402 (2010).
Niessen, F. et al. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature 452, 654–658 (2008).
Sun, X. et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am. J. Respir. Cell. Mol. Biol. 47, 628–636 (2012).
Schulze, T. et al. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 25, 4024–4036 (2011).
Allende, M. L. et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286, 7348–7358 (2011).
Urbano, M. et al. SAR analysis of innovative selective small molecule antagonists of sphingosine-1-phosphate 4 (S1P4) receptor. Bioorg. Med. Chem. Lett. 21, 5470–5474 (2011).
Jenne, C. N. et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 206, 2469–2481 (2009).
Mattes, H. et al. Design and synthesis of selective and potent orally active S1P5 agonists. ChemMedChem 5, 1693–1696 (2010).
Yang, L. et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J. Hepatol. 59, 114–123 (2013).
Kapitonov, D. et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 69, 6915–6923 (2009).
Price, M. M. et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent mouse model of allergic asthma. J. Allergy Clin. Immunol. 131, 501–511 (2012).
Dickson, M. A. et al. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin. Cancer Res. 17, 2484–2492 (2011).
Gao, P., Peterson, Y. K., Smith, R. A. & Smith, C. D. Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS ONE 7, e44543 (2012).
Antoon, J. W. et al. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. J. Mol. Endocrinol. 46, 205–216 (2011).
Liu, Q. et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS ONE 7, e41834 (2012).
Finney, C. A. et al. S1P is associated with protection in human and experimental cerebral malaria. Mol. Med. 17, 717–725 (2011).
Scuto, A. et al. STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res. 71, 3182–3188 (2011).
Saddoughi, S. A. et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 5, 105–121 (2013).
Shimizu, H. et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation 111, 222–229 (2005).
Suzuki, C. et al. Efficacy of mycophenolic acid combined with KRP-203, a novel immunomodulator, in a rat heart transplantation model. J. Heart Lung Transplant. 25, 302–309 (2006).
Song, J. et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J. Pharmacol. Exp. Ther. 324, 276–283 (2008).
Wenderfer, S. E., Stepkowski, S. M. & Braun, M. C. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 74, 1319–1326 (2008).
Poti, F. et al. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R−/−) mice. Vascul. Pharmacol. 57, 56–64 (2012).
Zhang, Z. Y. et al. AUY954, a selective S1P1 modulator, prevents experimental autoimmune neuritis. J. Neuroimmunol. 216, 59–65 (2009).
Galicia-Rosas, G. et al. A sphingosine-1-phosphate receptor 1-directed agonist reduces central nervous system inflammation in a plasmacytoid dendritic cell-dependent manner. J. Immunol. 189, 3700–3706 (2012).
Lien, Y. H., Yong, K. C., Cho, C., Igarashi, S. & Lai, L. W. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 69, 1601–1608 (2006).
Awad, A. S. et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 79, 1090–1098 (2011).
Jo, E. et al. S1P1-selective in vivo-active agonists from high- throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem. Biol. 12, 703–715 (2005).
Nishi, T. et al. Discovery of CS-0777: a potent, selective, and orally active S1P1 agonist. ACS Med. Chem. Lett. 2, 368–372 (2011).
Marsolais, D. et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc. Natl Acad. Sci. USA 106, 1560–1565 (2009).
Fujii, Y. et al. Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J. Immunol. 188, 206–215 (2012).
Gonzalez-Cabrera, P. J. et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-Like headgroup interactions. Mol. Pharmacol. 74, 1308–1318 (2008).
Davis, M. D., Clemens, J. J., Macdonald, T. L. & Lynch, K. R. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 280, 9633–9641 (2005).
Tarrason, G. et al. The sphingosine-1-phosphate receptor-1 antagonist, W146, causes early and short-lasting peripheral blood lymphopenia in mice. Int. Immunopharmacol. 11, 1773–1779 (2011).
Finley, A. et al. Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PLoS ONE 8, e55255 (2013).
Kluk, M. J. et al. Sphingosine-1-phosphate receptor 1 in classical Hodgkin lymphoma: assessment of expression and role in cell migration. Lab. Invest. 93, 462–471 (2013).
Foss, F. W. Jr et al. Synthesis and biological evaluation of γ-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg. Med. Chem. 15, 663–677 (2007).
Skoura, A. et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 81–85 (2011).
Ikeda, H. et al. Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology 124, 459–469 (2003).
Bolli, M. H. et al. 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J. Med. Chem. 53, 4198–4211 (2010).
Visentin, B. et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9, 225–238 (2006).
Caballero, S. et al. Anti-sphingosine-1-phosphate monoclonal antibodies inhibit angiogenesis and sub-retinal fibrosis in a murine model of laser-induced choroidal neovascularization. Exp. Eye Res. (2008).
Sanada, Y. et al. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS ONE 6, e23933 (2011).
Clemens, J. J., Davis, M. D., Lynch, K. R. & Macdonald, T. L. Synthesis of benzimidazole based analogues of sphingosine-1-phosphate: discovery of potent, subtype-selective S1P4 receptor agonists. Bioorg. Med. Chem. Lett. 14, 4903–4906 (2004).
Ota, H. et al. S1P4 receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm. Circ. 1, 399–404 (2011).
Song, J., Hagiya, H., Kurata, H., Mizuno, H. & Ito, T. Prevention of GVHD and graft rejection by a new S1P receptor agonist, W-061, in rat small bowel transplantation. Transpl. Immunol. 26, 163–170 (2012).
Acknowledgements
Work in the laboratory of S.S. is supported by grants R37GM043880 and RO1CA61774 from the US National Institutes of Health (NIH). The work of G.T.K. is supported by the NIH grant T32 HL094290.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Angiogenesis
-
The development of new blood vessels. Angiogenesis is required in development and tissue repair, as well as pathologically for tumour progression.
- Fingolimod
-
A sphingosine-1-phosphate receptor (S1PR) agonist. However, sustained activation of S1PR1 by fingolimod leads to degradation of S1PR1. Thus, fingolimod is often referred to as a 'functional antagonist' of S1PR1, particularly in its role in lymphocyte trafficking.
- Histone deacetylases
-
(HDACs). Proteins that remove acetyl groups from specific histone lysine residues, thus altering gene transcription.
- ABC transporters
-
(ATP-binding cassette transporters). A family of proteins that transport small molecules across the membrane, including drugs and lipids. Several of these proteins have been shown to transport sphingosine-1-phosphate.
- 'Inside-out' signalling
-
A model whereby agonists such as growth factors promote the production of sphingosine-1-phosphate (S1P) within the cell. This S1P is then exported outside the cell to signal through cell surface S1P receptors in an autocrine and/or paracrine manner.
- Lymphopenia
-
An abnormally low level of lymphocytes in the blood.
- Sphingolipid rheostat concept
-
A concept that describes how the metabolic balance between sphingosine-1-phosphate (S1P) and ceramide regulates cell fate. S1P-mediated signals mostly regulate cell survival and proliferation, whereas ceramide-mediated signals regulate growth inhibition and apoptosis.
- Myeloid cell
-
A blood cell type that includes macrophages, monocytes, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes and dendritic cells but not lymphocytes.
- Lymphangiogenesis
-
The development of new lymph vessels. Lymphangiogenesis is required in development and for tissue repair, as well as for the metastasis of some tumours, such as breast cancer tumours.
- Sjögren–Larsson syndrome
-
An autosomal recessive form of ichthyosis (scaly, dry, thickened skin) that is characterized by spastic paraplegia and mild to moderate intellectual disability.
- Ichthyosis
-
A family of mostly genetic skin disorders characterized by dry, thickened, scaly skin, often with cracks.
- mdx mice
-
A mouse model of Duchenne muscular dystrophy, which is a muscle-wasting disease that is caused by a mutation in the X-linked dystrophin gene, leading to loss of expression of the dystrophin protein.
- Plaque psoriasis
-
An autoimmune disorder of the skin in which the patient typically presents with scaly patches of skin with a red and/or white hue.
- Bradycardia
-
A decreased resting heart rate that results in in dizziness, weakness and fatigue.
- Macular oedema
-
The accumulation of fluid and protein in the macula (visual field), leading to swelling and loss of vision.
- Experimental autoimmune encephalomyelitis
-
(EAE). An animal model of inflammation-induced demyelinating disease, often used as a proxy for human multiple sclerosis.
- Cytokine storm
-
A potentially fatal immune reaction consisting of a positive feedback loop between highly elevated levels of many cytokines with immune cells.
- Sprouting angiogenesis
-
The process of developing new blood vessels in which angiogenic factors bind to receptors on endothelial cells of a blood vessel. These cells grow out and form sprouts connecting to other blood vessels.
- Laminar shear stress
-
The stress on tissues derived from the flow of a fluid through the vessel.
- Osteoporosis
-
A bone-thinning disease that is characterized by overactive bone resorption by osteoclasts, reduced bone formation by osteoblasts, or both.
- Sepsis syndrome
-
A life-threatening systemic response to severe infection that is characterized by vascular leakage and oedema, hypo- or hyperthermia, low blood pressure and reduced lung function.
- Dendritic cell
-
A cell type that has a central role in the adaptive immune response by presenting antigens to lymphocytes and causing their activation.
- TH17 cell
-
T helper 17 cell; a subset of T helper cells that secrete pro-inflammatory cytokines.
Rights and permissions
About this article
Cite this article
Kunkel, G., Maceyka, M., Milstien, S. et al. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12, 688–702 (2013). https://doi.org/10.1038/nrd4099
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4099
This article is cited by
-
Phase I study of opaganib, an oral sphingosine kinase 2-specific inhibitor, in relapsed and/or refractory multiple myeloma
Annals of Hematology (2023)
-
Enhanced selective capture of phosphomonoester lipids enabling highly sensitive detection of sphingosine 1-phosphate
Analytical and Bioanalytical Chemistry (2023)
-
S1PR1 regulates ovarian cancer cell senescence through the PDK1-LATS1/2-YAP pathway
Oncogene (2023)
-
Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway
Leukemia (2022)
-
Synchronous Investigation of the Mechanism and Substance Basis of Tripterygium Glycosides Tablets on Anti-rheumatoid Arthritis and Hepatotoxicity
Applied Biochemistry and Biotechnology (2022)