Abstract
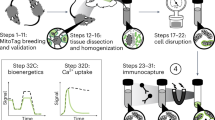
Mitochondria are not only the 'powerhouse' of the cell; they are also involved in a multitude of processes that include calcium storage, the cell cycle and cell death. Traditional means of investigating mitochondrial importance in a given cellular process have centered upon depletion of mtDNA through chemical or genetic means. Although these methods severely disrupt the mitochondrial electron transport chain, mtDNA-depleted cells still maintain mitochondria and many mitochondrial functions. Here we describe a straightforward protocol to generate mammalian cell populations with low to nondetectable levels of mitochondria. Ectopic expression of the ubiquitin E3 ligase Parkin, combined with short-term mitochondrial uncoupler treatment, stimulates widespread mitophagy and effectively eliminates mitochondria. In this protocol, we explain how to generate Parkin-expressing, mitochondria-depleted cells from scratch in 23 d, as well as offer a variety of methods for confirming mitochondrial clearance. Furthermore, we describe culture conditions to maintain mitochondrial-depleted cells for up to 30 d with minimal loss of viability, for longitudinal studies. This method should prove useful for investigating the importance of mitochondria in a variety of biological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 12, 34 (2014).
Pickrell, A.M. & Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273 (2015).
Dorn, G.W. II, Vega, R.B. & Kelly, D.P. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 29, 1981–1991 (2015).
Youle, R.J. & Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 (2011).
Quiros, P.M., Langer, T. & Lopez-Otin, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 16, 345–359 (2015).
Riley, J.S. & Tait, S.W. Mechanisms of mitophagy: putting the powerhouse into the doghouse. Biol. Chem. 397, 617–635 (2016).
Jin, S.M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010).
Yamano, K. & Youle, R.J. PINK1 is degraded through the N-end rule pathway. Autophagy 9, 1758–1769 (2013).
Kane, L.A. et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143–153 (2014).
Koyano, F. et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162–166 (2014).
Kazlauskaite, A. et al. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460, 127–139 (2014).
Chen, Y. & Dorn, G.W. II. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475 (2013).
Gong, G. et al. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 350, aad2459 (2015).
Narendra, D., Tanaka, A., Suen, D.-F. & Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Tait, S.W. et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 5, 878–885 (2013).
Correia-Melo, C. et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 35, 724–742 (2016).
King, M.P. & Attardi, G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 264, 304–313 (1996).
Kukat, A. et al. Generation of rho0 cells utilizing a mitochondrially targeted restriction endonuclease and comparative analyses. Nucleic Acids Res. 36, e44 (2008).
Jazayeri, M. et al. Inducible expression of a dominant negative DNA polymerase-gamma depletes mitochondrial DNA and produces a rho0 phenotype. J. Biol. Chem. 278, 9823–9830 (2003).
Jacobson, M.D. et al. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 361, 365–369 (1993).
Tait, S.W. & Green, D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 (2010).
Tait, S.W., Ichim, G. & Green, D.R. Die another way—non-apoptotic mechanisms of cell death. J. Cell Sci. 127, 2135–2144 (2014).
Kim, Y.S., Morgan, M.J., Choksi, S. & Liu, Z.G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 26, 675–687 (2007).
Vanlangenakker, N. et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 18, 656–665 (2011).
Cho, Y.S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
van Deursen, J.M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Correia-Melo, C. & Passos, J.F. Mitochondria: are they causal players in cellular senescence? Biochim. Biophys. Acta 1847, 1373–1379 (2015).
Passos, J.F. et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 6, 347 (2010).
Metcalf, D.J., Garcia-Arencibia, M., Hochfeld, W.E. & Rubinsztein, D.C. Autophagy and misfolded proteins in neurodegeneration. Exp. Neurol. 238, 22–28 (2012).
Rosenfeldt, M.T. & Ryan, K.M. The multiple roles of autophagy in cancer. Carcinogenesis 32, 955–963 (2011).
Baudot, A.D., Haller, M., Mrschtik, M., Tait, S.W. & Ryan, K.M. Using enhanced-mitophagy to measure autophagic flux. Methods 75, 105–111 (2015).
Padman, B.S., Bach, M., Lucarelli, G., Prescott, M. & Ramm, G. The protonophore CCCP interferes with lysosomal degradation of autophagic cargo in yeast and mammalian cells. Autophagy 9, 1862–1875 (2013).
Pear, W.S., Nolan, G.P., Scott, M.L. & Baltimore, D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90, 8392–8396 (1993).
Swift, S., Lorens, J., Achacoso, P. & Nolan, G.P. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Current Protocols in Immunology (eds. Coligan J.E. et al.) Chapter 10 Unit 10.17C, http://dx.doi.org/10.1002/0471142735.im1017cs31 (2001).
Hayflick, L. & Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Minamikawa, T., Williams, D.A., Bowser, D.N. & Nagley, P. Mitochondrial permeability transition and swelling can occur reversibly without inducing cell death in intact human cells. Exp. Cell Res. 246, 26–37 (1999).
Yoshii, S.R., Kishi, C., Ishihara, N. & Mizushima, N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 286, 19630–19640 (2011).
Chan, N.C. et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 20, 1726–1737 (2011).
Acknowledgements
We thank J. Long and K. Ryan (Cancer Research UK Beatson Institute, Glasgow, United Kingdom) for providing KP-4 cells expressing Parkin. We thank G.P. Nolan (Stanford University, Stanford, California, USA) for providing the pLZRS-empty vector plasmid. C.C.-M. was funded by Newcastle University and the Foundation for Science and Technology (FCT) through the GABBA Programme, University of Porto, Porto, Portugal. G.I. was supported by an EMBO advanced long-term postdoctoral fellowship (aALTF 772–2015). This research was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) (grant BB/K008374/1) and the Royal Society (both to S.W.G.T.). S.W.G.T. is a Royal Society University Research Fellow. Work in the J.F.P. laboratory was funded by a David Phillips Fellowship (BB/H022384/1) and a (BBSRC grant BB/K017314/1). We thank C. Winchester (Beaston Institute, Glasgow, Scotland) for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
C.C.-M., G.I., S.W.G.T. and J.F.P. developed the protocol. C.C.-M., G.I., S.W.G.T. and J.F.P. designed the experiments described herein. G.I. and C.C.-M. performed the experiments, interpreted the results and prepared figures. C.C.-M., G.I., S.W.G.T. and J.F.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Correia-Melo, C., Ichim, G., Tait, S. et al. Depletion of mitochondria in mammalian cells through enforced mitophagy. Nat Protoc 12, 183–194 (2017). https://doi.org/10.1038/nprot.2016.159
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.159
This article is cited by
-
Exploring the limitations of mitochondrial dye as a genuine horizontal mitochondrial transfer surrogate
Communications Biology (2024)
-
Apoptotic stress causes mtDNA release during senescence and drives the SASP
Nature (2023)
-
Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis
Cell Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.