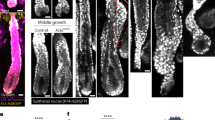
Abstract
Hair follicles are mammalian skin organs that periodically and stereotypically regenerate from a small pool of stem cells. Hence, hair follicles are a widely studied model for stem cell biology and regeneration. This protocol describes the use of two-photon laser-scanning microscopy (TPLSM) to study hair regeneration within a living, uninjured mouse. TPLSM provides advantages over conventional approaches, including enabling time-resolved imaging of single hair follicle stem cells. Thus, it is possible to capture behaviors including apoptosis, proliferation and migration, and to revisit the same cells for in vivo lineage tracing. In addition, a wide range of fluorescent reporter mouse lines facilitates TPLSM in the skin. This protocol also describes TPLSM laser ablation, which can spatiotemporally manipulate specific cellular populations of the hair follicle or microenvironment to test their regenerative contributions. The preparation time is variable depending on the goals of the experiment, but it generally takes 30–60 min. Imaging time is dependent on the goals of the experiment. Together, these components of TPLSM can be used to develop a comprehensive understanding of hair regeneration during homeostasis and injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Morrison, S.J. & Spralding, A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Rompolas, P. & Greco, V. Stem cell dynamics in the hair follicle niche. Sem. Cell Dev. Biol. 25–26, 34–42 (2014).
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nat. Lett. 457, 92–96 (2008).
Xie, Y. et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nat. Lett. 457, 97–101 (2008).
Yoshida, S., Sukeno, M. & Nabeshima, Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722–1726 (2007).
Uchugonova, A., Hoffman, R.M., Weinigel, M. & Koenig, K. Watching stem cells in the skin of living mice noninvasively. Cell Cycle 10, 2017–2020 (2011).
Li, J.L., Goh, C.C., Keeble, J.L. & Win, J.S. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Fuchs, E. Finding one's niche in the skin. Cell Stem Cell 4, 499–502 (2009).
Fuchs, E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell Rev. 137, 811–819 (2009).
Cotsarelis, G. Epithelial stem cells: a folliculocentric view. J. Investig. Dermatol. 126, 1459–1468 (2006).
Li, L. & Clevers, H. Coexistence of quiescence and active adult stem cells in mammals. Science 327, 542–545 (2010).
Cotsarelis, G., Sun, R.R. & Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Jahoda, C.A., Horne, K.A. & Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562 (1984).
Muller-Rover, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 117, 3–15 (2001).
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009).
Plikus, M.V. et al. Cyclic dermal BMP signaling regulates stem cell activation during hair regeneration. Nat. Lett. 451, 340–344 (2008).
Page, M.E., Lomard, P., Ng, F., Gottgens, B. & Jensen, K.B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482 (2013).
Chen, X., Nadiarynkh, O., Plotnikov, S. & Campagnola, P.J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 7, 654–659 (2012).
Campagnola, P.J. & Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues, and organisms. Nat. Biotechnol. 21, 1356–1360 (2003).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Rendl, M., Lewis, L. & Fuchs, E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 3, 1910–1924 (2005).
Kwan, K.M. Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis 32, 49–62 (2002).
Sauer, B. & Henderson, N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 85, 5166–5170 (1988).
Stemberg, N. & Hamilton, D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 150, 467–486 (1981).
Nagy, A. Cre recombinase: the universal reagent for genome tailoring. Genesis 26, 99–109 (2000).
Feil, R. et al. Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA 93, 10887–10890 (1996).
Feil, R., Wagner, J., Metzger, D. & Chambon, P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752–757 (1997).
Metzger, D., Clifford, J., Chiba, H. & Chambon, P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc. Natl. Acad. Sci. USA 92, 6991–6995 (1995).
Zhang, Y. et al. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 24, 543–548 (1996).
Youssef, K.K. et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 12, 299–305 (2010).
Means, A.L., Xu, Y., Zhao, A., Ray, K.C. & Gu, G. A CK19 (CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis 46, 318–323 (2008).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nat. Cell Biol. 449, 1003–1007 (2007).
Rompolas, P., Mesa, K. & Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 502, 513–518 (2013).
Cox, G. et al. 3-dimensional imaging of collagen using second harmonic generation. J. Struct. Biol. 141, 53–62 (2003).
Madisen, L., Zwingman, T.A. & Sunkin, S.M. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Dassule, H.R., Lewis, P., Bei, M., Maas, R & McMahon, AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785 (2000).
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA 96, 8551–8556 (1999).
Snippert, H. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
Vitale-Cross, L., Amornphimoltham, P., Fisher, G., Molinolo, A.A. & Gutkind, J.S. Conditional expression of K-ras in an epithelial compartment that includes the stem cells is sufficient to promote squamous cell carcinogenesis. Cancer Res. 64, 8804–8807 (2004).
Acknowledgements
This work is supported by The New York Stem Cell Foundation and grants to V.G. by the American Cancer Society, grant no. RSG-12-059-02; Yale Spore Grant National Cancer Institute, US National Institutes of Health (NIH) grant no. 1P50CA121974; and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIH grant no. 1R01AR063663-01. C.M.P. is supported by the Human Genetics Training Grant (HGTG) through Yale University. S.P. is supported by the James Hudson Brown-Alexander Brown Coxe Postdoctoral Fellowship. K.R.M. is supported by the NIH Predoctoral Program in Cellular and Molecular Biology, grant no. 5T32 GM007223, and is currently a National Science Foundation (NSF) Graduate Research Fellow. A.M.H. is supported by NIAMS Rheumatic Diseases Research Core Centers, grant no. 5 P30 AR053495-07. P.R. is a New York Stem Cell Foundation Druckenmiller Fellow and is supported by the CT Stem Cell Grant 13-SCA-YALE-20. V.G. is a New York Stem Cell Foundation Robertson Investigator. The authors gratefully acknowledge the laboratory of E. Fuchs (Rockefeller University) for providing the K14H2BGFP mice.
Author information
Authors and Affiliations
Contributions
C.M.P., S.P. and P.R. wrote the manuscript and prepared the figures. K.R.M. conducted experiments for figures. M.W., D.G.G. and A.M.H. helped develop and troubleshoot the TPLSM technique. V.G. oversaw and assisted all aspects of the protocol.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Hair follicle regeneration captured in vivo
Serial optical sections of a hair follicle in early growth as seen using a K14H2BGFP reporter. Collagen-rich extracellular matrix can be seen through second harmonic regeneration (SHG; blue channel). See also Figure 2. (MOV 2102 kb)
Cell proliferation captured during hair follicle growth.
Time-resolved intravital imaging demonstrating a magnified view of the lower portion of the hair follicle during the growth (anagen) phase of the hair cycle where epithelial cell migration can be observed using a K14H2BGFP reporter. (MOV 2185 kb)
Cell migration captured during hair follicle growth.
Time-resolved intravital imaging of epithelial cell migration as seen by nuclear displacement using a K14H2BGFP reporter. (MOV 2319 kb)
Cell death captured during hair follicle regression.
Time-resolved intravital imaging demonstrating apoptosis of epithelial cells as seen by nuclear fragmentation using a K14H2BGFP reporter; occurring during the regression (catagen) phase of the hair cycle. (MOV 318 kb)
Laser ablation of the hair follicle mesenchymal niche.
Serial optical sections of a hair follicle before and after laser ablation of the mesenchymal dermal papilla niche as seen using a mesenchymal (Lef1RFP; red channel) and epithelial (K14H2BGFP; green channel) reporter. Collagen-rich extracellular matrix can be seen through second harmonic regeneration (SHG; blue channel). Note the autofluorescent “halo” surrounding the ablation site. See also Figure 6. (MOV 5362 kb)
Rights and permissions
About this article
Cite this article
Pineda, C., Park, S., Mesa, K. et al. Intravital imaging of hair follicle regeneration in the mouse. Nat Protoc 10, 1116–1130 (2015). https://doi.org/10.1038/nprot.2015.070
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.070
This article is cited by
-
Dedifferentiation maintains melanocyte stem cells in a dynamic niche
Nature (2023)
-
Intravital microscopy of dynamic single-cell behavior in mouse mammary tissue
Nature Protocols (2021)
-
Skin-resident immune cells actively coordinate their distribution with epidermal cells during homeostasis
Nature Cell Biology (2021)
-
Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice
Nature Cell Biology (2017)
-
Correction of aberrant growth preserves tissue homeostasis
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.