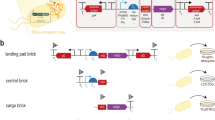
Abstract
Here we describe an advanced paradigm for the design, construction and stable implementation of complex biological systems in microbial organisms. This engineering strategy was previously applied to the development of an Escherichia coli–based platform, which enabled the use of brown macroalgae as a feedstock for the production of biofuels and renewable chemicals. In this approach, functional genetic modules are first designed in silico and constructed on a bacterial artificial chromosome (BAC) by using a recombineering-based inchworm extension technique. Stable integration into the recipient chromosome is then mediated through the use of recombinase-assisted genome engineering (RAGE). The flexibility, simplicity and speed of this method enable a comprehensive optimization of several different parameters, including module configuration, strain background, integration locus, gene copy number and intermodule compatibility. This paradigm therefore has the potential to markedly expedite most strain-engineering endeavors. Once a biological system has been designed and constructed on a BAC, its implementation and optimization in a recipient host can be carried out in as little as 1 week.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tabor, J.J. et al. A synthetic genetic edge detection program. Cell 137, 1272–1281 (2009).
Nandagopal, N. & Elowitz, M.B. Synthetic biology: integrated gene circuits. Science 333, 1244–1248 (2011).
Uchiyama, T. & Miyazaki, K. Functional metagenomics for enzyme discovery: challenges to efficient screening. Curr. Opin. Biotechnol. 20, 616–622 (2009).
Gagarinova, A. & Emili, A. Genome-scale genetic manipulation methods for exploring bacterial molecular biology. Mol. BioSystems 8, 1626–1638 (2012).
Felnagle, E.A., Chaubey, A., Noey, E.L., Houk, K.N. & Liao, J.C. Engineering synthetic recursive pathways to generate non-natural small molecules. Nat. Chem. Biol. 8, 518–526 (2012).
Lee, J.W. et al. Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat. Chem. Biol. 8, 536–546 (2012).
Peralta-Yahya, P.P., Zhang, F., del Cardayre, S.B. & Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 488, 320–328 (2012).
Santos, C.N.S. & Stephanopoulos, G. Combinatorial engineering of microbes for optimizing cellular phenotype. Curr. Opin. Chem. Biol. 12, 168–176 (2008).
Henry, C.S., Broadbelt, L.J. & Hatzimanikatis, V. Discovery and analysis of novel metabolic pathways for the biosynthesis of industrial chemicals: 3-hydroxypropanoate. Biotechnol. Bioeng. 106, 462–473 (2010).
Gibson, D.G. et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 319, 1215–1220 (2008).
Kodumal, S.J. et al. Total synthesis of long DNA sequences: synthesis of a contiguous 32-kb polyketide synthase gene cluster. Proc. Natl. Acad. Sci. USA 101, 15573–15578 (2004).
Fu, J. et al. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446 (2012).
Quan, J. & Tian, J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE 4, e6441 (2009).
Gibson, D.G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Li, M.Z. & Elledge, S.J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 4, 251–256 (2007).
Alper, H., Fischer, C., Nevoigt, E. & Stephanopoulos, G. Tuning genetic control through promoter engineering. Proc. Natl. Acad. Sci. USA 102, 12678–12683 (2005).
Pfleger, B.F., Pitera, D.J., Smolke, C.D. & Keasling, J.D. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat. Biotechnol. 24, 1027–1032 (2006).
Salis, H.M., Mirsky, E.A. & Voigt, C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 27, 946–950 (2009).
Mutalik, V.K. et al. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat. Methods 10, 354–360 (2013).
Birnbaum, S. & Bailey, J.E. Plasmid presence changes the relative levels of many host cell proteins and ribosome components in recombinant Escherichia coli. Biotechnol. Bioeng. 37, 736–745 (1991).
Jones, K.L., Kim, S.W. & Keasling, J.D. Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab. Eng. 2, 328–338 (2000).
Tyo, K.E., Ajikumar, P.K. & Stephanopoulos, G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat. Biotechnol. 27, 760–765 (2009).
Bentley, W.E. & Quiroga, O.E. Investigation of subpopulation heterogeneity and plasmid stability in recombinant Escherichia coli via a simple segregated model. Biotechnol. Bioeng. 42, 222–234 (1993).
Paulsson, J. & Ehrenberg, M. Noise in a minimal regulatory network: plasmid copy number control. Q. Rev. Biophys. 34, 1–59 (2001).
Santos, C.N.S., Regitsky, D.D. & Yoshikuni, Y. Implementation of stable and complex biological systems through recombinase-assisted genome engineering. Nat. Commun. 4, 2503 (2013).
Wargacki, A.J. et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335, 308–313 (2012).
Sharan, S.K., Thomason, L.C., Kuznetsov, S.G. & Court, D.L. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4, 206–223 (2009).
Wang, H.H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009).
Monaco, A.P. & Larin, Z. YACs, BACs, PACs and MACs: artificial chromosomes as research tools. Trends Biotechnol. 12, 280–286 (1994).
Yan, X., Yu, H.J., Hong, Q. & Li, S.P. Cre/lox system and PCR-based genome engineering in Bacillus subtilis. Appl. Environ. Microbiol. 74, 5556–5562 (2008).
Yu, B.J. & Kim, C. Minimization of the Escherichia coli genome using the Tn5-targeted Cre/loxP excision system. Methods Mol. Biol. 416, 261–277 (2008).
Datsenko, K.A. & Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Song, C.W. & Lee, S.Y. Rapid one-step inactivation of single or multiple genes in Escherichia coli. Biotechnol. J. 8, 776–784 (2013).
Yu, D., Sawitzke, J.A., Ellis, H. & Court, D.L. Recombineering with overlapping single-stranded DNA oligonucleotides: testing a recombination intermediate. Proc. Natl. Acad. Sci. USA 100, 7207–7212 (2003).
Yu, D. et al. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97, 5978–5983 (2000).
Haldimann, A. & Wanner, B.L. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183, 6384–6393 (2001).
Boyd, D., Weiss, D.S., Chen, J.C. & Beckwith, J. Towards single-copy gene expression systems making gene cloning physiologically relevant: λ InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182, 842–847 (2000).
Rodionov, D.A., Gelfand, M.S. & Hugouvieux-Cotte-Pattat, N. Comparative genomics of the KdgR regulon in Erwinia chrysanthemi 3937 and other γ-proteobacteria. Microbiology 150, 3571–3590 (2004).
Lee, G. & Saito, I. Role of nucleotide sequences of loxP spacer region in Cre-mediated recombination. Gene 216, 55–65 (1998).
Langer, S.J., Ghafoori, A.P., Byrd, M. & Leinwand, L. A genetic screen identifies novel non-compatible loxP sites. Nucleic Acids Res. 30, 3067–3077 (2002).
Siegel, R.W., Jain, R. & Bradbury, A. Using an in vivo phagemid system to identify non-compatible loxP sequences. FEBS Lett. 499, 147–153 (2001).
Glaser, S., Anastassiadis, K. & Stewart, A.F. Current issues in mouse genome engineering. Nat. Genet. 37, 1187–1193 (2005).
Louwerse, J.D. et al. Stable recombinase-mediated cassette exchange in Arabidopsis using Agrobacterium tumefaciens. Plant Physiol. 145, 1282–1293 (2007).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Construction of pALG3 plasmid for alginate degradation and metabolism.
1: pALG1 was first constructed by screening a fosmid library of the Vibrio splendidus 12B01 genome. We found several other genetic components that might be responsible for the degradation and metabolism of alginate downstream of the segment found in pALG1 in its genome. 2-4: Two genes downstream of the genomic segment found in pALG1 (V12B01_24254 and V12B01_24259) were cloned in the pKD13-based plasmid. The integration cassette was prepared by PCR (2) and integrated into pALG1 using recombineering techniques (3). The kanamycin selection marker was excised with a FLP recombinase (4) to yield pALG1.1. 5-7: Three other genes downstream of the genomic segment found in pALG1.1 (V12B01_24264, V12B01_24269, and V12B01_24274) were cloned in the pKD13-based plasmid. The integration cassette was prepared by PCR (5) and integrated into pALG1.1 using recombineering techniques (6). The kanamycin selection marker was excised with a FLP recombinase (7) to yield pALG1.2. 8 and 9: Two other genetic components were found downstream of the genomic segment in pALG1.2 (V12B01_24309 and V12B01_24324) and cloned in the pKm-based plasmid. The integration cassette was prepared by PCR (8), and the cassette was integrated into pALG1.2 using recombineering techniques (9) to yield pALG3.
Supplementary Figure 2 Construction of pALG7 plasmid for alginate and cellobiose degradation and metabolism.
1: We found several other genetic components that might be responsible for the degradation and metabolism of alginate downstream of the genomic segment in pALG1 and in the genomes of other microbes such as Agrobacterium tumefaciens C58 and Pseudoalteromonas sp. SM0524. We also found genes that may be responsible for cellobiose degradation and metabolism in Saccharophagus degradans 2-40. These genetic components were integrated into the module. 2-3: Three genetic components were found downstream of the genomic segment in pALG1 (V12B01_24309, V12B01_24324, and V12B01_24269) and cloned in the pKm-based plasmid (pKm_V12B01_24309_24324_24269). The integration cassette was prepared by PCR (2) and integrated into pALG1 using recombineering techniques (3) to yield pALG2. 4-5: An operon comprising genetic components Atu_3019-3026 (suspected to be responsible for alginate metabolism in Agrobacterium tumefaciens C58) was cloned in the pCm-based plasmid (pCm_Atu2019-3026). The integration cassette was prepared using PCR (4) and integrated into pALG2 using recombineering techniques (5) to yield pALG3.0. 6-8: Two genes downstream of the genomic segment found in pALG1 (V12B01_24254 and V12B01_24259) were cloned in the pKD13-based plasmid (pKD_V12B01_24254-24259). The integration cassette was prepared by PCR (6) and integrated into pALG3.0 using recombineering techniques (7). The kanamycin selection marker was excised via FLP recombinase (8) to yield pALG3.5. 9-10: Genetic components that were suspected to be responsible for cellobiose metabolism in Saccharophagus degradans 2-40 were cloned in the pKm-based plasmid (pKm_Sdes). The integration cassette was prepared with PCR (9) and integrated into pALG3.5 using recombineering techniques (10) to yield pALG4.0. 11-12: A gene encoding a bifunctional alginate lyase derived from Pseudoalteromonas sp. SM0524 was engineered to be secretable in E. coli and was cloned in pBeloBAC11-based plasmid (pCm and pBeloBAC11 share the chloramphenicol resistance gene). This integration cassette was prepared by PCR (11) and integrated into pALG4.0 using recombineering techniques (12) to yield pALG7.2. 13-15; three other genes downstream of the genomic segment found in pALG1 (V12B01_24264, V12B01_24269, and V12B01_24274) were cloned in the pKD13-based plasmid (pKD_V12B01_24264-24274). The integration cassette was prepared by PCR (13). This cassette was integrated into pAL7.2 using recombineering techniques (14). The kanamycin selection marker was excised via FLP recombinase (15) to yield pALG7.8.
Supplementary information
Supplementary Figure 1
Construction of pALG3 plasmid for alginate degradation and metabolism. (PDF 341 kb)
Supplementary Figure 2
Construction of pALG7 plasmid for alginate and cellobiose degradation and metabolism. (PDF 363 kb)
Rights and permissions
About this article
Cite this article
Santos, C., Yoshikuni, Y. Engineering complex biological systems in bacteria through recombinase-assisted genome engineering. Nat Protoc 9, 1320–1336 (2014). https://doi.org/10.1038/nprot.2014.084
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.084
This article is cited by
-
Creating custom synthetic genomes in Escherichia coli with REXER and GENESIS
Nature Protocols (2021)
-
CRAGE enables rapid activation of biosynthetic gene clusters in undomesticated bacteria
Nature Microbiology (2019)
-
An integrated workflow for phenazine-modifying enzyme characterization
Journal of Industrial Microbiology and Biotechnology (2018)
-
Genome reprogramming for synthetic biology
Frontiers of Chemical Science and Engineering (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.