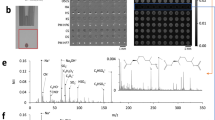
Abstract
Glycosaminoglycans (GAGs) possess considerable heterogeneity in average molecular mass, molecular mass range, disaccharide composition and content and position of sulfo groups. Despite recent technological advances in the analysis of GAGs, the determination of GAG disaccharide composition still remains challenging and provides key information required for understanding GAG function. Analysis of GAG-derived disaccharides relies on enzymatic treatment, providing one of the most practical and quantitative approaches for compositional mapping. Tagging the reducing end of disaccharides with an aromatic fluorescent label affords stable derivatives with properties that enable improved detection and resolution. HPLC with on-line electrospray ionization mass spectrometry (ESI-MS) offers a relatively soft ionization method for detection and characterization of sulfated oligosaccharides. GAGs obtained from tissues, biological fluids or cells are treated with various enzymes to obtain disaccharides that are fluorescently labeled with 2-aminoacridone (AMAC) and resolved by different LC systems for high-sensitivity detection by fluorescence, and then they are unambiguously characterized by MS. The preparation and labeling of GAG-derived disaccharides can be performed in ∼1–2 d, and subsequent HPLC separation and on-line fluorescence detection and ESI-MS analysis takes another 1–2 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gupta, G. & Surolia, A. Glycomics: an overview of the complex glycocode. Adv. Exp. Med. Biol. 749, 1–13 (2012).
Raman, R. et al. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat. Methods 2, 817–824 (2005).
Shriver, Z. et al. Glycomics: a pathway to a class of new and improved therapeutics. Nat. Rev. Drug Discov. 3, 863–873 (2004).
Dennis, J.W. et al. Protein glycosylation in development and disease. Bioessays 21, 412–421 (1999).
Feizi, T. & Mulloy, B. Carbohydrates and glycoconjugates. Glycomics: the new era of carbohydrate biology. Curr. Opin. Struct. Biol. 13, 602–604 (2003).
Ly, M. et al. Proteoglycanomics: recent progress and future challenges. OMICS 14, 389–399 (2010).
Gesslbauer, B. et al. Proteoglycanomics: tools to unravel the biological function of glycosaminoglycans. Proteomics 7, 2870–2880 (2007).
Morgan, M.R. et al. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957–969 (2007).
Capila, I. & Linhardt, R.J. Heparin–protein interactions. Angew. Chem. Int. Ed. Engl. 41, 391–412 (2002).
Cattaruzza, S. & Perris, R. Approaching the proteoglycome: molecular interactions of proteoglycans and their functional output. Macromol. Biosci. 6, 667–680 (2006).
Cattaruzza, S. et al. Proteoglycans in the control of tumor growth and metastasis formation. Connect. Tissue Res. 49, 225–229 (2008).
Gesslbauer, B. et al. New targets for glycosaminoglycans and glycosaminoglycans as novel targets. Exp. Rev. Proteomics 10, 77–95 (2013).
Zhao, X. et al. Sequence analysis and domain motifs in the porcine skin decorin glycosaminoglycan chain. J. Biol. Chem. 288, 9226–9237 (2013).
Li, L. et al. Proteoglycan sequence. Mol. Biosyst. 8, 1613–1625 (2012).
Jackson, R.L. et al. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol. Rev. 71, 481–539 (1991).
Gama, C.I. & Hsieh-Wilson, L.C. Chemical approaches to deciphering the glycosaminoglycan code. Curr. Opin. Chem. Biol. 9, 609–619 (2005).
Fraser, J.R.E. et al. Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 242, 27–33 (1997).
Volpi, N. Analytical aspects of pharmaceutical grade chondroitin sulfates. J. Pharm. Sci. 96, 3168–3180 (2007).
Sugahara, K. et al. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 13, 612–620 (2003).
Yamada, S. & Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug. Discov. Technol. 5, 289–301 (2008).
Sugahara, K. & Mikami, T. Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 17, 536–545 (2007).
Volpi, N. Advances in chondroitin sulfate analysis: application in physiological and pathological states of connective tissue and during pharmacological treatment of osteoarthritis. Curr. Pharm. Des. 12, 639–658 (2006).
Linhardt, R.J. et al. New methodologies in heparin structure analysis and the generation of LMW heparins. Adv. Exp. Med. Biol. 313, 37–47 (1992).
Rabenstein, D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 19, 312–331 (2002).
Casu, B. Structure of heparin and heparin fragments. Ann. N.Y. Acad. Sci. 556, 1–17 (1989).
Casu, B. Structure and biological activity of heparin. Adv. Carbohydr. Chem. Biochem. 43, 51–134 (1985).
Kamhi, E. et al. Glycosaminoglycans in infectious disease. Biol. Rev. Camb. Philos. Soc. 88, 928–943 (2013).
Kogan, G. et al. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 29, 17–25 (2007).
Volpi, N. et al. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 16, 1718–1745 (2009).
Gray, E. et al. The anticoagulant and antithrombotic mechanisms of heparin. Handb. Exp. Pharmacol. 207, 43–61 (2012).
Hassan, Y. et al. Heparin-induced thrombocytopenia and recent advances in its therapy. J. Clin. Pharm. Ther. 32, 535–544 (2007).
Masuko, S. & Linhardt, R.J. Chemoenzymatic synthesis of the next generation of ultralow MW heparin therapeutics. Future Med. Chem. 4, 289–296 (2012).
Wang, Z et al. Control of the heparosan N-deacetylation leads to an improved bioengineered heparin. Appl. Microbiol. Biotechnol. 91, 91–99 (2011).
Zhou, H. et al. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PLoS ONE 6, e21106 (82011).
Volpi, N. (Ed.) Chondroitin Sulfate: Structure, Role, and Pharmacological Activity (Academic Press, 2006).
Karst, N.A. & Linhardt, R.J. Recent chemical and enzymatic approaches to the synthesis of glycosaminoglycan oligosaccharides. Curr. Med. Chem. 10, 1993–2031 (2003).
Leymarie, N. & Zaia, J. Effective use of mass spectrometry for glycan and glycopeptide structural analysis. Anal. Chem. 84, 3040–3048 (2012).
Volpi, N. et al. Electrophoresis for the analysis of heparin purity and quality. Electrophoresis 33, 1531–1537 (2012).
Yang, B. et al. Hyphenated techniques for the analysis of heparin and heparan sulfate. Anal. Bioanal. Chem. 399, 541–557 (2011).
Sisu, E. et al. Modern developments in mass spectrometry of chondroitin and dermatan sulfate glycosaminoglycans. Amino Acids 41, 235–256 (2011).
Beni, S. et al. Analysis and characterization of heparin impurities. Anal. Bioanal. Chem. 399, 527–239 (2011).
Zaia, J. Mass spectrometry and glycomics. OMICS 14, 401–418 (2010).
Zaia, J. On-line separations combined with MS for analysis of glycosaminoglycans. Mass. Spectrom. Rev. 28, 254–272 (2009).
Volpi, N. et al. Capillary electrophoresis of complex natural polysaccharides. Electrophoresis 29, 3095–3106 (2008).
Amon, S. et al. Glycosylation analysis of glycoproteins and proteoglycans using capillary electrophoresis-mass spectrometry strategies. Electrophoresis 29, 2485–2507 (2008).
Volpi, N. & Linhardt, R.J. High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides. Nat. Protoc. 5, 993–1004 (2010).
Michaud, P. et al. Polysaccharide lyases: recent developments as biotechnological tools. Crit. Rev. Biotechnol. 23, 233–266 (2003).
Ernst, S. et al. Enzymatic degradation of glycosaminoglycans. Crit. Rev. Biochem. Mol. Biol. 30, 387–444 (1995).
Linhardt, R.J. et al. Polysaccharide lyases. Appl. Biochem. Biotechnol. 12, 135–176 (1986).
Xia, B. et al. Versatile fluorescent derivatization of glycans for glycomic analysis. Nat. Methods 2, 845–850 (2005).
Hase, H. Precolumn derivatization for chromatographic and electrophoretic analyses of carbohydrates. J. Chrom. A 720, 173–182 (1996).
Chang, Y. et al. Capillary electrophoresis for the analysis of glycosaminoglycan-derived disaccharides. Methods Mol. Biol. 984, 67–77 (2013).
Chang, Y. et al. Analysis of glycosaminoglycan-derived disaccharides by capillary electrophoresis using laser-induced fluorescence detection. Anal. Biochem. 427, 91–98 (2012).
Yang, B. et al. Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 1225, 91–98 (2012).
Galeotti, F. & Volpi, N. Online reverse phase-high-performance liquid chromatography-fluorescence detection-electrospray ionization-mass spectrometry separation and characterization of heparan sulfate, heparin, and low-molecular weight-heparin disaccharides derivatized with 2-aminoacridone. Anal. Chem. 83, 6770–6777 (2011).
Takegawa, Y. et al. Simultaneous analysis of heparan sulfate, chondroitin/dermatan sulfates, and hyaluronan disaccharides by glycoblotting-assisted sample preparation followed by single-step zwitterionic-hydrophilic interaction chromatography. Anal. Chem. 83, 9443–9449 (2011).
Skidmore, M.A. et al. Disaccharide compositional analysis of heparan sulfate and heparin polysaccharides using UV or high-sensitivity fluorescence (BODIPY) detection. Nat. Protoc. 5, 1983–1992 (2010).
Volpi, N. High-performance liquid chromatography and on-line mass spectrometry detection for the analysis of chondroitin sulfates/hyaluronan disaccharides derivatized with 2-aminoacridone. Anal. Biochem. 397, 12–23 (2010).
Ambrosius, M. et al. Quantitative determination of the glycosaminoglycan δ-disaccharide composition of serum, platelets and granulocytes by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1201, 54–60 (2008).
Deakin, J.A. et al. Simplified and sensitive fluorescent method for disaccharide analysis of both heparan sulfate and chondroitin/dermatan sulfates from biological samples. Glycobiology 18, 483–491 (2008).
Hitchcock, A.M. et al. Improved workup for glycosaminoglycan disaccharide analysis using CE with LIF detection. Electrophoresis 29, 4538–4548 (2008).
Militsopoulou, M. et al. Determination of twelve heparin- and heparan sulfate-derived disaccharides as 2-aminoacridone derivatives by capillary zone electrophoresis using ultraviolet and laser-induced fluorescence detection. Electrophoresis 23, 1104–1109 (2002).
Okafo, G. et al. A coordinated high-performance liquid chromatographic, capillary electrophoretic, and mass spectrometric approach for the analysis of oligosaccharide mixtures derivatized with 2-aminoacridone. Anal. Chem. 68, 4424–4430 (1996).
Starr, C.M. et al. Fluorophore-assisted carbohydrate electrophoresis in the separation, analysis, and sequencing of carbohydrates. J. Chromatogr. A 720, 295–321 (1996).
Imanari, T. et al. High-performance liquid chromatographic analysis of glycosaminoglycan-derived oligosaccharides. J. Chromatogr. A 720, 275–293 (1996).
Galeotti, F. & Volpi, N. Novel reverse-phase ion pair-high performance liquid chromatography separation of heparin, heparan sulfate and low molecular weight-heparins disaccharides and oligosaccharides. J. Chromatogr. A 1284, 141–147 (2013).
Karamanos, N.K. et al. Ion-pair high-performance liquid chromatography for determining disaccharide composition in heparin and heparan sulphate. J. Chromatogr. A 765, 169–179 (1997).
Calabro, A. et al. Fluorophore-assisted carbohydrate electrophoresis (FACE) of glycosaminoglycans. Osteoarthritis Cartilage 9, S16–S22 (2001).
Okafo, G. et al. A coordinated high-performance liquid chromatographic, capillary electrophoretic, and mass spectrometric approach for the analysis of oligosaccharide mixtures derivatized with 2-aminoacridone. Anal. Chem. 68, 4424–4430 (1996).
Zhang, Z. et al. Liquid chromatography-mass spectrometry to study chondroitin lyase action pattern. Anal. Biochem. 385, 57–64 (2009).
Linhardt, R.J. Analysis of glycosaminoglycans with polysaccharide lyases. Curr. Protoc. Mol. Biol. 48, 17.13.B.1–17.13.B.16 (2001).
Buzzega, D. et al. Fluorophore-assisted carbohydrate electrophoresis for the determination of molecular mass of heparins and low-molecular-weight (LMW) heparins. Electrophoresis 29, 4192–4202 (2008).
Volpi, N. & Maccari, F. Detection of submicrogram quantities of glycosaminoglycans on agarose gels by sequential staining with toluidine blue and Stains-All. Electrophoresis 23, 4060–4066 (2002).
Zhang, F. et al. Microscale isolation and analysis of heparin from plasma using an anion exchange column. Anal. Biochem. 353, 284–286 (2006).
Shao, C. et al. Comparative glycomics of leukocyte glycosaminoglycans. FEBS J. 280, 2447–2461 (2013).
Di Iorio, E. et al. Localization and expression of CHST6 and keratan sulfate proteoglycans in the human cornea. Exp. Eye Res. 91, 293–299 (2010).
Mizumoto, S. & Sugahara, K. Glycosaminoglycan chain analysis and characterization (glycosylation/epimerization). Methods Mol. Biol. 836, 99–115 (2012).
Cesaretti, M. et al. A 96-well assay for uronic acid carbazole reaction. Carb. Polym. 54, 59–61 (2003).
Malavaki, C.J. et al. Capillary electrophoresis for the quality control of chondroitin sulfates in raw materials and formulations. Anal. Biochem. 374, 213–220 (2008).
Korir, A.K. et al. Ultraperformance ion-pair liquid chromatography coupled to electrospray time-of-flight mass spectrometry for compositional profiling and quantification of heparin and heparan sulfate. Anal. Chem. 80, 1297–1306 (2008).
Saad, O.M. & Leary, J.A. Compositional analysis and quantification of heparin and heparan sulfate by electrospray ionization ion trap mass spectrometry. Anal. Chem. 75, 2985–2995 (2003).
Zhang, Z. et al. Tandem MS can distinguish hyaluronic acid from N-acetylheparosan. J. Am. Soc. Mass Spectrom. 19, 82–90 (2008).
Kailemia, M.J. et al. Complete mass spectral characterization of a synthetic ultralow-molecular-weight heparin using collision-induced dissociation. Anal. Chem. 84, 5475–5478 (2012).
Galeotti, F. et al. On-line high-performance liquid chromatography-fluorescence detection-electrospray ionization-mass spectrometry profiling of human milk oligosaccharides derivatized with 2-aminoacridone. Anal. Biochem. 430, 97–104 (2012).
Lawrence, R. et al. Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat. Methods 5, 291–292 (2008).
Da Col, R. et al. Characterization of the chemical structure of sulphated glycosaminoglycans after enzymatic digestion. Application of liquid chromatography-mass spectrometry with an atmospheric pressure interface. J. Chromatogr. 647, 289–300 (1993).
Oguma, T. et al. Analytical method of chondroitin/dermatan sulfates using high performance liquid chromatography/turbo ionspray ionization mass spectrometry: application to analyses of the tumor tissue sections on glass slides. Biomed. Chromatogr. 15, 356–362 (2001).
Oguma, T. et al. Analytical method of heparan sulfates using high-performance liquid chromatography turbo-ionspray ionization tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 754, 153–159 (2001).
Zaia, J. & Costello, C.E. Compositional analysis of glycosaminoglycans by electrospray mass spectrometry. Anal. Chem. 73, 233–239 (2001).
Hitchcock, A.M. et al. Glycoform quantification of chondroitin/dermatan sulfate using a liquid chromatography-tandem mass spectrometry platform. Biochemistry 45, 2350–2361 (2006).
Kuberan, B. et al. Analysis of heparan sulfate oligosaccharides with ion pair-reverse phase capillary high performance liquid chromatography-microelectrospray ionization time-of-flight mass spectrometry. J. Am. Chem. Soc. 124, 8707–8718 (2002).
Kühn, A.V. et al. Quantification of hyaluronic acid fragments in pharmaceutical formulations using LC-ESI-MS. J. Pharm. Biomed. Anal. 30, 1531–1537 (2003).
Thanawiroon, C. et al. Liquid chromatography/mass spectrometry sequencing approach for highly sulfated heparin-derived oligosaccharides. J. Biol. Chem. 279, 2608–2615 (2004).
Volpi, N. Mass spectrometry characterization of Escherichia coli K4 oligosaccharides from 2-mers to more than 20-mers. Rapid Commun. Mass Spectrom. 21, 3459–3466 (2007).
Volpi, N. et al. Mass spectrometry for the characterization of unsulfated chondroitin oligosaccharides from 2-mers to 16-mers. Comparison with hyaluronic acid oligomers. Rapid Commun. Mass Spectrom. 22, 3526–3530 (2008).
Volpi, N. Chondroitin C lyase [4.2.2.] is unable to cleave fructosylated sequences inside the partially fructosylated Escherichia coli K4 polymer. Glycoconj. J. 25, 451–457 (2008).
Volpi, N. & Maccari, F. Structural characterization and antithrombin activity of dermatan sulfate purified from marine clam Scapharca inaequivalvis. Glycobiology 19, 356–367 (2009).
Henriksen, J. et al. On-line size-exclusion chromatography/mass spectrometry of low-molecular-mass heparin. J. Mass Spectrom. 39, 1305–1312 (2004).
Barroso, B. et al. Analysis of proteoglycans derived sulphated disaccharides by liquid chromatography/mass spectrometry. J. Chromatogr. A 1080, 43–48 (2005).
Karlsson, N.G. et al. Use of graphitised carbon negative ion LC-MS to analyse enzymatically digested glycosaminoglycans. J. Chromatogr. B 824, 139–147 (2005).
Henriksen, J. et al. Ion-pairing reversed-phased chromatography/mass spectrometry of heparin. Carb. Res. 341, 382–387 (2006).
Bisio, A. et al. High-performance liquid chromatographic/mass spectrometric studies on the susceptibility of heparin species to cleavage by heparanase. Semin. Throm. Hemost. 33, 488–495 (2007).
Volpi, N. On-line HPLC/ESI-MS separation and characterization of hyaluronan oligosaccharides from 2-mers to 40-mers. Anal. Chem. 79, 6390–6397 (2007).
Naimy, H. et al. Characterization of heparin oligosaccharides binding specifically to antithrombin III using mass spectrometry. Biochemistry 47, 3155–3161 (2008).
Hitchcock, A.M. et al. Comparative glycomics of connective tissue glycosaminoglycans. Proteomics 8, 1384–1397 (2008).
Doneanu, C.E. et al. Analysis of oligosaccharides derived from heparin by ion-pair reversed-phase chromatography/mass spectrometry. Anal. Chem. 81, 3485–3499 (2009).
Zhang, Z. et al. Quantification of heparan sulfate disaccharides using ion-pairing reversed-phase microflow high-performance liquid chromatography with electrospray ionization trap mass spectrometry. Anal. Chem. 81, 4349–4355 (2009).
Heinegard, D. Hyaluronidase digestion and alkaline treatment of bovine tracheal cartilage proteoglycans. Biochem. Biophys. Acta 285, 193–207 (1972).
Brustkern, A.M. et al. Characterization of currently marketed heparin products: reversed-phase ion-pairing liquid chromatography mass spectrometry of heparin digests. Anal. Chem. 82, 9865–9870 (2012).
Yang, B. et al. Ultra-performance ion-pairing liquid chromatography with on-line electrospray ion trap mass spectrometry for heparin disaccharide analysis. Anal. Biochem. 415, 59–66 (2011).
Wang, B. et al. Characterization of currently marketed heparin products: analysis of heparin digests by RPIP-UHPLC-QTOF-MS. J. Pharm. Biomed. Anal. 67-68, 42–50 (2012).
Li, L. et al. Top-down approach for the direct characterization of low-molecular-weight heparins using LC-FT-MS. Anal. Chem. 84, 8822–8829 (2013).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work. N.V. designed and developed the on-line HPLC-FD-ESI-MS analysis of AMAC-tagged GAGs disaccharides. R.J.L. and B.Y. designed and developed the RP-HPLC-ESI-MS concurrent separation of multiple families of AMAC-tagged GAG disaccharides. F.G. applied this methodology to the structural study of various GAGs.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Tuning of mass spectral parameters to minimize sulfo-group loss on N,2,6triSHS disaccharide (D2S6).
A. Spectra showing a high sulfo-group (SO3) loss. B. Optimized spectra after tuning showing no (or minimal) sulfo group (SO3) loss. This tuning should be performed prior to performing analyses.
Supplementary information
Supplementary Figure 1
Tuning of mass spectral parameters to minimize sulfo-group loss on N,2,6triSHS disaccharide (D2S6). (PDF 164 kb)
Rights and permissions
About this article
Cite this article
Volpi, N., Galeotti, F., Yang, B. et al. Analysis of glycosaminoglycan-derived, precolumn, 2-aminoacridone–labeled disaccharides with LC-fluorescence and LC-MS detection. Nat Protoc 9, 541–558 (2014). https://doi.org/10.1038/nprot.2014.026
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.026
This article is cited by
-
Identification of global inhibitors of cellular glycosylation
Nature Communications (2023)
-
Glycosaminoglycan signatures in body fluids of mucopolysaccharidosis type II mouse model under long-term enzyme replacement therapy
Journal of Molecular Medicine (2022)
-
Discrimination of sulfated isomers of chondroitin sulfate disaccharides by HILIC-MS
Analytical and Bioanalytical Chemistry (2021)
-
Proteoglycan serglycin promotes non-small cell lung cancer cell migration through the interaction of its glycosaminoglycans with CD44
Journal of Biomedical Science (2020)
-
Glycosaminoglycan Domain Mapping of Cellular Chondroitin/Dermatan Sulfates
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.