Abstract
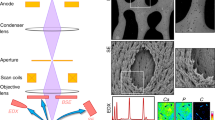
In this protocol, we describe a 3D imaging technique known as 'volume electron microscopy' or 'focused ion beam scanning electron microscopy (FIB/SEM)' applied to biological tissues. A scanning electron microscope equipped with a focused gallium ion beam, used to sequentially mill away the sample surface, and a backscattered electron (BSE) detector, used to image the milled surfaces, generates a large series of images that can be combined into a 3D rendered image of stained and embedded biological tissue. Structural information over volumes of tens of thousands of cubic micrometers is possible, revealing complex microanatomy with subcellular resolution. Methods are presented for tissue processing, for the enhancement of contrast with osmium tetroxide/potassium ferricyanide, for BSE imaging, for the preparation and platinum deposition over a selected site in the embedded tissue block, and for sequential data collection with ion beam milling; all this takes ∼90 h. The imaging conditions, procedures for alternate milling and data acquisition and techniques for processing and partitioning the 3D data set are also described; these processes take ∼30 h. The protocol is illustrated by application to developing chick cornea, in which cells organize collagen fibril bundles into complex, multilamellar structures essential for transparency in the mature connective tissue matrix. The techniques described could have wide application in a range of fields, including pathology, developmental biology, microstructural anatomy and regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Sartori, A. et al. Correlative microscopy: bridging the gap between fluorescence light microscopy and cryo-electron tomography. J. Struct. Biol. 160, 135–145 (2007).
Verkade, P. Moving EM: the Rapid Transfer System as a new tool for correlative light and electron microscopy and high throughput for high-pressure freezing. J. Microsc. 230, 317–328 (2008).
Brown, E., Mantell, J., Carter, D., Tilly, G. & Verkade, P. Studying intracellular transport using high-pressure freezing and Correlative Light Electron Microscopy. Semin. Cell Dev. Biol. 20, 910–919 (2009).
Ngo, H. et al. Focused ion beam in dental research. Am. J. Dent. 13, 31D–34D (2000).
Nalla, R.K. et al. Ultrastructural examination of dentin using focused ion-beam cross-sectioning and transmission electron microscopy. Micron 36, 672–680 (2005).
FEI. Slice and View. http://www.fei.com/applications/life-sciences/tissue-biology/3d-tissue-imaging.aspx (2005).
Soto, G.E. et al. Serial section electron tomography: a method for three-dimensional reconstruction of large structures. Neuroimage 1, 230–243 (1994).
White, J., Southgate, E., Thomson, J. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. A. 314, 1–340 (1986).
Canty, E.G. et al. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 165, 553–563 (2004).
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004).
Armer, H.E. et al. Imaging transient blood vessel fusion events in zebrafish by correlative volume electron microscopy. PLoS One 4, 1–10 (2009).
Heymann, J.A. et al. Site-specific 3D imaging of cells and tissues with a dual beam microscope. J. Struct. Biol. 155, 63–73 (2006).
Hayles, M.F., Stokes, D.J., Phifer, D. & Findlay, K.C. A technique for improved ion beam milling of cryo-prepared life science specimens. J. Microsc. 226, 263–269 (2007).
Knott, G., Marchman, H., Wall, D. & Lich, B. Serial section scanning electron microscopy of adult brain tissue. J. Neorosci. 28, 2959–2964 (2008).
Hekking, L.H.P. et al. Focused ion beam-scanning electron microscope: exploring large volumes of atherosclerotic tissue. J. Microsc. 235, 336–347 (2009).
De Winter, D.A.M. et al. Tomography of insulating biological and geological materials using focused ion beam (FIB) sectioning and low-kv BSE imaging. J. Microsc. 233, 372–383 (2009).
Quantock, A.J. & Young, R.D. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev. Dyn. 237, 2607–2621 (2008).
Young, R.D. et al. Keratocyte-mediated establishment of lamellar structure in corneal development. Acta Opthalmol. 88; doi: 10.1111/j.1755-3768.2010.203.x (2010).
Abramoff, M.D., Magelhaes, P.J. & Ram, S.J. Image processing with ImageJ. Biophotonics Int. 11, 36–42 (2004).
Hamburger, V. & Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951).
Acknowledgements
Our cornea research at Cardiff University is supported by project grants from the EPSRC (EP/F034970 to A.J.Q. and R.D.Y.) and Biotechnology and Biological Sciences Resource Council (BB/F022077/1 to C.K. and A.J.Q.). The use of the FEI Quanta 3D microscope at Queen Mary University of London is funded by an Engineering and Physical Sciences Research Council Access to Equipment Scheme grant (EP/F019882/1 to A.J.B.).
Author information
Authors and Affiliations
Contributions
All authors wrote the paper. A.J.Q. designed the experimental series, of which this paper is a part, to elucidate corneal structural development. R.D.Y. carried out specimen preparation, light and transmission electron microscopy. K.M.Y.P. operated the Quanta FIB/SEM microscope, under A.J.B., supervision and collected the data sets for the 3D reconstruction. C.P. and C.K. carried out the image processing and 3D rendering.
Corresponding author
Ethics declarations
Competing interests
K.M.Y.P. is currently employed by Carl Zeiss NTS, a manufacturer of FIB/SEM instruments. K.M.Y.P. does not hold shares in Carl Zeiss NTS. The remaining authors declare that they have no competing financial interests.
Supplementary information
Supplementary Video 1
XY projection from 230 raw backscattered electron image sequence from FIB/SEM of 14 day embryonic chick cornea. (MOV 4817 kb)
Supplementary Video 2
XY projection from 230 image sequence from FIB/SEM of 14 day embryonic chick cornea after contrast reversal. (MOV 1328 kb)
Supplementary Video 3
3D reconstruction of corneal keratocytes in chick cornea at 14 day development with rotation about the y-axis showing lamellar arrangement of cells and filopodial extensions. (MOV 9045 kb)
Supplementary Video 4
3D reconstruction of corneal keratocytes in chick cornea at 14 day development with rotation about the x-axis, through an en face view of the block showing orthogonal organisation of cell filopodia through the tissue volume. (MOV 10991 kb)
Rights and permissions
About this article
Cite this article
Bushby, A., P'ng, K., Young, R. et al. Imaging three-dimensional tissue architectures by focused ion beam scanning electron microscopy. Nat Protoc 6, 845–858 (2011). https://doi.org/10.1038/nprot.2011.332
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.332
This article is cited by
-
The application and development of electron microscopy for three-dimensional reconstruction in life science: a review
Cell and Tissue Research (2024)
-
CellRemorph: A Toolkit for Transforming, Selecting, and Slicing 3D Cell Structures on the Road to Morphologically Detailed Astrocyte Simulations
Neuroinformatics (2023)
-
Localized corrosion induced surface modifications of Al-Cu-Li alloy studied by ToF-SIMS 3D imaging
npj Materials Degradation (2021)
-
A structure–function based approach to floc hierarchy and evidence for the non-fractal nature of natural sediment flocs
Scientific Reports (2021)
-
Reactive oxygen FIB spin milling enables correlative workflow for 3D super-resolution light microscopy and serial FIB/SEM of cultured cells
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.