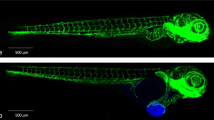
Abstract
Hypoxia facilitates tumor invasion and metastasis by promoting neovascularization and co-option of tumor cells in the peritumoral vasculature, leading to dissemination of tumor cells into the circulation. However, until recently, animal models and imaging technology did not enable monitoring of the early events of tumor cell invasion and dissemination in living animals. We recently developed a zebrafish metastasis model to dissect the detailed events of hypoxia-induced tumor cell invasion and metastasis in association with angiogenesis at the single-cell level. In this model, fluorescent DiI-labeled human or mouse tumor cells are implanted into the perivitelline cavity of 48-h-old zebrafish embryos, which are subsequently placed in hypoxic water for 3 d. Tumor cell invasion, metastasis and pathological angiogenesis are detected under fluorescent microscopy in the living fish. The average experimental time for this model is 7 d. Our protocol offers a remarkable opportunity to study molecular mechanisms of hypoxia-induced cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Erler, J.T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Rouhi, P. et al. Pathological angiogenesis facilitates tumor cell dissemination and metastasis. Cell Cycle 9, 913–917 (2010).
Cao, Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat. Rev. Cancer 5, 735–743 (2005).
Lee, S.L. et al. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc. Natl. Acad. Sci. USA 106, 19485–19490 (2009).
Nissen, L.J. et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J. Clin. Invest. 117, 2766–2777 (2007).
Cao, R. et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6, 333–345 (2004).
Cao, Z. et al. Hypoxia-induced retinopathy model in the adult zebrafish. Nat. Protoc., 12, 1903–1910 (2010).
van Rooijen, E. et al. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood 113, 6449–6460 (2009).
Lawson, N.D. & Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 (2002).
Jin, S.W., Beis, D., Mitchell, T., Chen, J.N. & Stainier, D.Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005).
Acknowledgements
Y.C.'s laboratory was supported by research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute distinguished professor award, the European Union Integrated Project of Metoxia (Project no. 222741) and the European Research Council (ERC) advanced grant ANGIOFAT (Project no 250021). Z.C. is supported by the Swedish Heart and Lung Foundation.
Author information
Authors and Affiliations
Contributions
Y.C. designed the study. L.D.J., P.R. and Z.C. performed the experiments. L.D.J., P.R., Z.C. and Y.C. analyzed the data. K.H., T.L., J.F.S. and E.W. participated in designing and discussing this study. L.D.J., P.R., Z.C. and Y.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rouhi, P., Jensen, L., Cao, Z. et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc 5, 1911–1918 (2010). https://doi.org/10.1038/nprot.2010.150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.150
This article is cited by
-
Zebrafish patient-derived xenograft models predict lymph node involvement and treatment outcome in non-small cell lung cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
Oxygen-sensitive methylation of ULK1 is required for hypoxia-induced autophagy
Nature Communications (2022)
-
Modeling oncolytic virus dynamics in the tumor microenvironment using zebrafish
Cancer Gene Therapy (2021)
-
TMBIM6/BI-1 contributes to cancer progression through assembly with mTORC2 and AKT activation
Nature Communications (2020)
-
Pharmacophore-guided discovery of CDC25 inhibitors causing cell cycle arrest and tumor regression
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.