Abstract
Chronic intermittent alcohol vapor exposure leads to increased dynorphin (DYN) A-like peptide expression and heightened kappa-opioid receptor (KOR) signaling in the central nucleus of the amygdala (CeA) and these neuroadaptive responses differentiate alcohol-dependent from non-dependent phenotypes. Important for therapeutic development efforts is understanding the nature of the stimulus that drives dependence-like phenotypes such as escalated alcohol self-administration. Accordingly, the present study examined the impact of intra-CeA KOR antagonism on escalated operant alcohol self-administration and physiological withdrawal symptoms during acute withdrawal and protracted abstinence in rats previously exposed to chronic intermittent alcohol vapor. Following operant training, rats were implanted with intra-CeA guide cannula and exposed to long-term intermittent alcohol vapor exposure that resulted in escalated alcohol self-administration and elevated physiological withdrawal signs during acute withdrawal. Animals received intra-CeA infusions of the KOR antagonist nor-binaltorphimine (nor-BNI; 0, 2, 4, or 6 μg) prior to operant alcohol self-administration sessions and physiological withdrawal assessment during acute withdrawal and protracted abstinence. The results indicated that site-specific KOR antagonism in the CeA ameliorated escalated alcohol self-administration during both acute withdrawal and protracted abstinence test sessions, whereas KOR antagonism had no effect on physiological withdrawal scores at either time point. These results dissociate escalated alcohol self-administration from physiological withdrawal symptoms in relation to KOR signaling in the CeA and help clarify the nature of the stimulus that drives escalated alcohol self-administration during acute withdrawal and protracted abstinence.
Similar content being viewed by others
Introduction
Alcohol abuse and dependence is ranked as the third leading preventable cause of death in the United States with approximately 88 000 deaths annually (Stahre et al, 2014). Particularly unnerving are data showing that 50–90% of the alcoholics who attempt abstinence, experience relapse (Charney et al, 2010). This chronic relapsing disorder is marked by aversive symptoms during acute withdrawal from alcohol that impede recovery and are thought to promote excessive alcohol consumption according to ‘self-medication’ hypotheses (Markou et al, 1998; Koob, 2009; Walker et al, 2012). In humans, common acute physiological withdrawal symptoms include sweating, increased heart rate, nausea, hyper-reflexia, agitation, fever, tremors, and convulsions (Hall and Zador, 1997), while protracted abstinence is associated with motivational deficits and persistent bouts of depression and anxiety that can last for months or years after alcohol cessation (Roelofs, 1985; De Soto et al, 1989). Similar to humans, symptoms of physiological withdrawal in rodents include tremors, hyperirritability, rigidity, increased susceptibility to audiogenic seizures, and presence of the ventromedial distal limb flexion response (Williams et al, 2012). Complementing existing animal models of alcohol dependence-like phenotypes, it was recently shown that alcohol-dependent rats reliably display negative affective-like states during acute withdrawal (Valdez and Harshberger, 2012; Williams et al, 2012; Berger et al, 2013; Kissler et al, 2014). It has been postulated that aversive symptoms of withdrawal are primary obstacles to reduced the intake of, or abstinence from, alcohol once dependent (Markou et al, 1998; Koob, 2009; Walker et al, 2012).
A promising avenue for treatment of alcohol abuse and dependence is through modulation of the kappa-opioid receptor (KOR) and its endogenous ligand, dynorphin (DYN). This system is expressed throughout the brain and is known to produce dysphoria in humans and aversive behaviors in rodents. KOR antagonists have shown efficacy for ameliorating excessive alcohol consumption during acute withdrawal in alcohol-dependent rats when administered systemically, centrally, and site-specifically in the nucleus accumbens (Walker and Koob, 2008; Nealey et al, 2011; Walker et al, 2011). It was recently identified that increased central amygdala (CeA) DYN expression and KOR function differentiate alcohol-dependent from non-dependent alcohol self-administration (Kissler et al, 2014). The CeA contains a dense population of KORs (Mansour et al, 1987) and is a functionally interconnected region of the extended amygdala that integrates emotional, learning, motivational, nociceptive, and decision-making information (Alheid and Heimer, 1988; Koob and Volkow, 2010; Sirohi et al, 2012).
An important question related to the ability of CeA KOR antagonism to reduce excessive alcohol self-administration during acute withdrawal is the nature of the stimulus being reduced by KOR blockade that results in diminished escalated alcohol self-administration. Given that the CeA integrates information from cortical, limbic, and brainstem nuclei subserving a variety of different functions (Alheid and Heimer, 1988; Koob and Volkow, 2010), it is possible that KOR antagonists or partial agonists reduce aversive symptoms of withdrawal hypothesized to drive escalated alcohol self-administration in alcohol-dependent rats (for review, see Spanagel, 2009). Considering that physiological withdrawal symptoms last for shorter periods of time compared with motivational, affective, and cognitive deficits that accompany alcohol dependence (Koob and Volkow, 2010; Elholm et al, 2011), an ideal goal for the treatment of alcohol use disorders would be a single pharmacotherapy that reduces alcohol consumption during both acute withdrawal and protracted abstinence. Accordingly, if KOR antagonist treatment in the CeA impacts physiological withdrawal symptoms to reduce escalated alcohol self-administration, one would predict that such efficacy would be short-lived (because physiological withdrawal is relatively fleeting). Such a limited timeframe for KOR antagonist efficacy could limit the utility of KOR antagonists to treat alcohol use disorders. Conversely, if intra-CeA KOR antagonism reduces escalated alcohol self-administration during acute withdrawal without impacting physiological withdrawal symptoms, initial support for a KOR-mediated dissociation of enhanced motivation for alcohol and physiological withdrawal would be established. Additional support for the dissociable effects of KOR antagonism on motivational and physiological withdrawal could be provided by evidence of escalated alcohol self-administration that persists into protracted abstinence and remains nor-BNI-sensitive. To directly assess these alternative hypotheses, the present study evaluated the effects of site-specific intra-CeA KOR antagonism (using multiple doses) for the ability to modulate operant alcohol self-administration and physiological withdrawal symptoms during acute withdrawal and protracted abstinence.
Methods
Animals
Male Wistar rats approximately 60 days old were pair- or triple-housed in an environmentally controlled vivarium with ad libitum food and water. Vivarium space was on a reversed light cycle (lights off at 6:00AM). Prior to operant training, all animals were handled daily for 1 week. All work adhered to the 2011 Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and followed Washington State University Institutional Animal Care and Use Committee guidelines.
Operant Alcohol Self-administration
Animals were trained to self-administer a 10% alcohol (w/v) solution using a sweetener-fade method (Walker and Koob, 2007) during daily 30 min operant sessions. Briefly, acquisition of the operant response occurred using a sweetened fluid (0.125% saccharin and 3% glucose) as the reinforcer. Next, 10% ethanol (w/v) was added to the solution while the sweetener was gradually removed over the course of 3 weeks until animals were self-administering a final solution of unadulterated 10% alcohol (w/v). Standard operant chambers (Med Associates, St. Albans, VT) with custom fluid wells (Behavioral Pharma, La Jolla, CA) were utilized, allowing the animals to press a single lever and receive 0.1 ml of solution (fixed-ratio 1 schedule of reinforcement). Individual stability of responding (defined as <10% deviation over two sessions with the average of the two sessions taken and used as the ‘baseline’ value in all data analyses) was required before intracranial surgeries.
Surgical Procedures
Animals were anesthetized with isoflurane gas (5% induction and ~2% for maintenance) and bilaterally implanted with guide cannulae targeting the CeA (anterior–posterior −2.3, medial–lateral ±4.2, dorsal–ventral −6.3 from bregma; (Paxinos and Watson, 2007). Guide cannulae were secured using dental acrylic and the open ends were sealed with obturators. All animals received postoperative care for 5 days consisting of Baytril (antibiotic), Flunixin (analgesic), and 0.9% sterile saline as needed.
Intermittent Alcohol Vapor Exposure
All animals were subjected to an intermittent alcohol vapor regimen that consisted of 14 h of vapor followed by 10 h of air exposure each day for an initial induction period of 4 weeks. This method has been shown to produce escalated alcohol self-administration, which is a hallmark of the alcohol dependence-like phenotype (Walker and Koob, 2008). Blood alcohol concentrations were analyzed biweekly via tail-bleed with samples collected before daily alcohol vapor termination and assessed using the Analox AM1 (Analox Instruments, Lunenberg, MA). Target blood alcohol concentrations of 175–225 mg% were maintained throughout the alcohol dependence component of the experiment and confirmed before operant testing during acute withdrawal.
Acute Withdrawal Self-administration and Physiological Withdrawal Measures
Following dependence induction, all animals self-administered alcohol twice per week during 30-min sessions in acute withdrawal (6–8 h after vapor termination) until stability was achieved (defined as <10% deviation over two sessions). Animals received sham and artificial cerebrospinal fluid (pH 7.2–7.4 was composed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 5.4 mM d-Glucose, and 0.25 mM ascorbic acid; Nealey et al, 2011) infusions (28 gauge internal cannula; Plastics One; Roanoke, VA) until stability was achieved for each step (see above). This served to habituate the animals to the infusion process prior to pharmacological manipulation and ensure that any changes in self-administration were due to the pharmacological manipulation. Following stable artificial cerebrospinal fluid-treated responding, animals received a single dose of the KOR antagonist nor-BNI (0, 2, 4, or 6 μg; n=5–6/group; Tocris Biosciences, Ellisville, MI), into the CeA. All infusions were 0.5 μl per side over 2 min with the internal cannula left in place for 1 min to allow diffusion of drug away from guide cannula. Pharmacological infusions took place 5 min prior to the final self-administration session during acute withdrawal (we have previously shown that nor-BNI pretreatments either 5 min or 24 h prior to testing produce complementary results on operant alcohol self-administration (Walker and Koob, 2008; Walker et al, 2011; Nealey et al, 2011)). Five days following self-administration testing, animals were tested during acute withdrawal for physiological withdrawal symptoms (Williams et al, 2012). Animals were weighed then placed in a clear standard rat cage without bedding. Animals were checked for the flexion response by grasping the nape of the neck and lifting up the animal and then were monitored for 3 min for irritability, tail rigidity, and abnormal gait and given a score ranging from 0 to 2 for each behavior with a score of 2 indicating severe or persistent presence of the symptom. Scores for all symptoms were combined to yield a single composite score for physiological withdrawal symptoms.
Protracted Abstinence Self-administration and Physiological Withdrawal Measures
Following physiological symptom measurement during acute withdrawal, animals were removed from the vapor chambers and exposed to an air-only environment for ~30 days. Animals remained alcohol-abstinent and were not tested in the operant chambers during this time period. On protracted abstinence self-administration test day, animals were placed into operant chambers and allowed to self-administer alcohol for 30 min during the time of day when acute withdrawal testing took place. Animals did not receive an additional infusion of nor-BNI prior to protracted abstinence testing because previous research has demonstrated an extended duration of action for classical KOR antagonists such as nor-BNI and JDTic to reduce behaviors related to alcohol consumption (eg, Walker et al, 2011; Deehan et al, 2012), the basis of which appears to be related to both c-Jun N-terminal kinase phosphorylation (Bruchas et al, 2007; Melief et al, 2011) and ligand accumulation in the brain (Patkar et al, 2013). Five days after self-administration testing, animals were tested for physiological withdrawal symptoms as described above.
Histology
Upon completion of the experiment, animals were killed and had their brains removed and stored in 4% formaldehyde at 4 °C until histological examination commenced using a Leica 1850 cryostat (Leica, Bannockburn, IL). Sections were mounted on slides and CeA placement was confirmed. Animals with correct unilateral placement were included after statistical analysis indicated no differences between bilateral and unilateral placement on self-administration responses, which allowed for unilateral and bilateral correct placement data to be pooled. Animals with misplaced guide cannulae were removed from statistical analysis with those animals having received nor-BNI infusions serving as negative controls.
Statistics
To confirm that chronic intermittent vapor exposure induced escalated self-administration, paired-sample t-tests were conducted on pre-vapor baseline and 1-month vapor exposure-induced alcohol self-administration. Confirmation that alcohol self-administration remained escalated at the time of protracted abstinence testing was achieved by conducting a paired-sample t-test on the nor-BNI vehicle group’s alcohol self-administration during the pre-dependence baseline compared with protracted abstinence alcohol self-administration. Separate univariate analyses of variance compared nor-BNI doses during acute withdrawal and protracted withdrawal with post hoc LSD tests conducted if a significant effect of dose was found. Because physiological withdrawal scores following alcohol vapor exposure were calculated using what many consider to be ordinal/categorical data, non-parametric independent-samples Kruskal–Wallis tests were used for the analyses with nor-BNI dose as the between-groups factor. To assess whether physiological withdrawal scores were attenuated in protracted abstinence compared with acute withdrawal, physiological withdrawal scores from the nor-BNI vehicle group during acute withdrawal were compared with scores collected during protracted abstinence using the non-parametric Repeated-Samples Wilcoxon Signed-Ranks Test.
Results
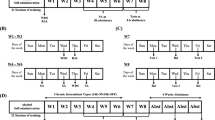
Eight animals were removed because both infusion sites were outside the CeA and two animals were removed from the study during the protracted abstinence-waiting period owing to complications. Of the eight animals removed owing to cannula misplacement, five received nor-BNI infusions and were used as a negative control group to evaluate the specificity of intra-CeA nor-BNI infusions (see Figure 1 for histology).
Histological confirmation of infusion sites targeting the CeA. Filled circles: • denote correct cannulae placement, whereas an ‘X’ indicates a nor-BNI-treated misplacement that was included in the negative control group. Note that for the sake of simplicity, the cannula sites depicted here show placements in only one hemisphere but are meant to reflect the bilateral locations of each cannula tip. Coronal sections taken from The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, 2005).
Alcohol-dependent rats assessed during acute withdrawal displayed significantly escalated alcohol self-administration when compared with pre-vapor exposure baseline (t (20)=−9.259; P <0.001); escalation that was significantly reduced by nor-BNI (main effect of dose, F (3, 17)=4.393, P=0.018; Figure 2). Post hoc LSD comparisons showed that the 2, 4, and 6 μg doses of nor-BNI were significantly different from vehicle (P=0.006; 0.010; 0.009, respectively). The negative control group did not differ from the vehicle-treated group (P>0.05), which demonstrates specificity of the intra-CeA nor-BNI infusions. During acute withdrawal, nor-BNI did not significantly alter physiological withdrawal scores (Kruskal–Wallis, H (3)=1.348, P=0.718; Figure 3).
Mean (+SEM) alcohol self-administration following chronic intermittent alcohol vapor exposure (represented by the solid line). Animals were tested during acute withdrawal and received a single dose of nor-BNI (0, 2, 4, or 6 μg; n=5/6 per group). Following dependence induction during acute withdrawal, alcohol self-administration was significantly escalated (δ=P<0.001). Nor-BNI attenuated escalated alcohol self-administration (main effect of dose, *P<0.05; **P⩽0.01 when compared with vehicle). aCSF, artificial cerebrospinal fluid; Base, baseline; NC, negative control.
Mean (+SEM) total physiological withdrawal scores during acute withdrawal. Nor-BNI did not alter physiological withdrawal measures when compared with vehicle controls. NC, negative control.
Following acute withdrawal symptom measurement, animals were subjected to ~30 days of abstinence from alcohol and re-tested during protracted abstinence for levels of operant alcohol self-administration and physiological withdrawal. The paired-sample t-test confirmed the continued presence of escalated alcohol self-administration in protracted withdrawal (t (4)=−3.325, P=0.029), whereas physiological withdrawal scores for the nor-BNI vehicle group were significantly lowered by the time of protracted withdrawal testing when compared with acute withdrawal while still alcohol-dependent (Wilcoxon Signed-Ranks Test, Z=15, P=0.038). The results of the univariate analyses of variance indicated a main effect of dose (Figure 4; F (3, 15)=6.287, P=0.006). Post hoc analyses yielded significant differences between vehicle and the 4 and 6 μg doses (P⩽0.05 and 0.01, respectively). The analysis of physiological withdrawal scores measured during protracted abstinence yielded a non-significant difference between doses of nor-BNI and vehicle (Kruskal–Wallis, H (3)=3.718, P=0.294; Figure 5).
Mean (+SEM) alcohol self-administration during protracted alcohol abstinence. Animals treated with nor-BNI (n=4–6/group) showed a dose-dependent reduction in escalated alcohol self-administration (main effect of dose, ‡‡P<0.01; *P⩽0.05 and **P<0.01 when compared with vehicle).
Mean (+SEM) total physiological withdrawal scores during protracted abstinence. Nor-BNI did not alter physiological withdrawal measures when compared with vehicle controls.
Discussion
The primary objective of the present study was to test the hypothesis that intra-CeA KOR antagonist-mediated reductions in escalated alcohol self-administration were dissociable from alterations in physiological withdrawal. Site-specific infusions of the KOR antagonist nor-BNI in the CeA attenuated escalated alcohol self-administration during acute withdrawal without impacting physiological withdrawal symptoms. Importantly, escalated alcohol self-administration persisted into protracted abstinence and was dose-dependently rescued by intra-CeA nor-BNI infusions, whereas physiological symptoms of withdrawal were significantly reduced during protracted abstinence when compared with acute withdrawal. The acute withdrawal and protracted abstinence data converge to support the hypothesis that intra-CeA KOR antagonism reduces escalated alcohol self-administration in a manner distinct from reductions in symptoms of physiological withdrawal and identify dysregulation of the DYN/KOR system in the CeA persists long term to promote maladaptive behavioral regulation in protracted abstinence.
In humans and rodents, withdrawal from alcohol is marked by aversive physiological symptoms and persistent motivational, affective and cognitive deficits (eg, Schuckit et al, 1997; Zhao et al, 2007; Williams et al, 2012). These aversive states have been posited to drive excessive alcohol consumption and promote relapse to alcohol abuse (Markou et al, 1998; Koob, 2009; Walker, 2012). Furthermore, central KOR activation induces negative affective-like behavior and has been implicated as a mediator of dysphoria associated with stress (Land et al, 2008), whereas KOR antagonists show anti-depressant efficacy (Mague et al, 2003) and ameliorate dependence and withdrawal-induced escalation of alcohol self-administration and increased negative affective-like behavior (Walker and Koob, 2008; Nealey et al, 2011; Walker et al, 2011; Berger et al, 2013). It was recently identified that alcohol dependence and withdrawal-induced increases in KOR function and DYN A-like peptide expression in the CeA contributes to escalated alcohol self-administration during acute withdrawal (Kissler et al, 2014).
Given that previous studies investigating the role of DYN/KORs in alcohol dependence have primarily focused on acute withdrawal-related phenotypes, a principal question has remained unanswered regarding the nature of the stimulus that drives escalated alcohol self-administration. The present data indicate that in the CeA, KOR antagonist efficacy for reducing escalated alcohol self-administration is dissociable from KOR antagonist effects on physiological withdrawal. Identifying that KOR activation in the CeA mediates escalated self-administration in a manner dissociable from physiological withdrawal symptoms is an important step towards understanding the nature of CeA KOR activation-induced escalation of alcohol self-administration. Other scenarios, such as altered positive reinforcing effects of alcohol, altered thirst, or sedation (as well as others; for review, see Walker, 2012), could explain reductions in escalated alcohol self-administration following intra-CeA KOR antagonist challenge, although many potential alternatives are not supported by the literature. For example, nor-BNI has no impact on water self-administration in alcohol-dependent rats (Walker and Koob, 2008; Walker et al, 2011), suggesting that altered thirst did not contribute to the effects of nor-BNI in the present study. Human and animal evidence points to dysregulated DYN/KORs as a basis for compromised motivation, mood, and executive function following chronic exposure to alcohol (Nealey et al, 2011; Bazov et al, 2013; Kissler et al, 2014). This hypothesis is supported by evidence showing that during withdrawal in alcohol-dependent animals, systemic and central KOR antagonism reduces negative affective-like behavior (Valdez and Harshberger, 2012; Berger et al, 2013) at doses that also reduce escalated alcohol self-administration (Walker and Koob, 2008; Walker et al, 2011) and intra-CeA KOR antagonism attenuates anxiety-like behavior in cocaine-withdrawn animals (Kallupi et al, 2013). Taken together, the CeA could be a potential substrate for KOR antagonist-induced reductions in withdrawal-induced negative affective-like behavior, with future research needed to test such a hypothesis.
One characteristic of nor-BNI that warrants reconciliation relates to nor-BNI selectivity for KORs vs μ-opioid receptors (MORs). Evidence from mice suggests that following administration, nor-BNI has mild MOR affinity that lasts at least 2 h (Broadbear et al, 1994) and could confound interpretation of the present results. However, the transient MOR affinity of nor-BNI that has been observed in mice was not replicated using rats (Picker et al, 1996). Furthermore, our published data from Wistar rats have confirmed that there are no observed differences in the effects of nor-BNI when administered 5 min or 24 h prior to alcohol self-administration sessions in nondependent and ethanol-dependent rats (Walker and Koob, 2008; Nealey et al., 2011; Walker et al, 2011). Additionally, if nor-BNI treatment occurred immediately prior to self-administration sessions and nor-BNI did have functionally relevant MOR affinity, then one would predict reduced alcohol self-administration in non-dependent rats as has been observed previously using selective MOR antagonists (eg, Hyytia, 1993). Indeed, following intra-CeA infusions of nor-BNI immediately prior to self-administration sessions, non-dependent alcohol self-administration was unaffected, whereas targeting intra-CeA MOR/δ-opioid receptors with subtype-selective antagonists did reduce non-dependent self-administration of alcohol (Kissler et al, 2014). Therefore, intra-CeA KOR and MOR/δ-opioid receptors antagonism had dissociable effects on alcohol self-administration in non-dependent Wistar rats that support nor-BNI’s KOR-selective profile in the present study.
Important for KOR-directed therapeutic development is the observation that escalated alcohol self-administration persisted into protracted (~30 days) abstinence following an extended alcohol vapor exposure period (~120 days) and remained KOR-sensitive. Given the dysregulation in the CeA DYN/KOR system that was previously observed during acute withdrawal (Kissler et al, 2014), the present data suggest that such dysregulation endures and continues to drive escalated alcohol self-administration. Indeed, the KOR antagonist nor-BNI dose-dependently reduced escalated alcohol self-administration during protracted abstinence. This is the first functional demonstration of protracted dysregulation in CeA DYN/KORs and extends the therapeutic potential of KOR antagonist efficacy beyond acute withdrawal. An important observation is that while escalated self-administration persisted into protracted abstinence, symptoms of physiological withdrawal did not persist which provides additional support for distinct substrates underlying motivational vs physiological withdrawal. During protracted abstinence, physiological withdrawal scores returned to levels observed in alcohol-naïve animals (Williams et al, 2012), which was not unexpected because physiological withdrawal symptoms persist no longer than 7–10 days on average (Elholm et al, 2011), a fact that serves as an internal control for withdrawal score assessment. An interesting feature of the present dataset was the lack of a nor-BNI dose–response curve during acute withdrawal that was restored in protracted abstinence. One could speculate that the lack of a dose–response could be explained by dependence-induced increases in CeA DYN A-like peptide expression combined with increased KOR function (Kissler et al, 2014) that allow the dysregulated system to partially overcome the effects of the lower nor-BNI doses used in the present study. Also likely during the waiting period for protracted abstinence testing are two time-dependent processes: (i) dissipation of nor-BNI effects over time with small doses such as the 2 μg dose falling below the ‘threshold’ for altering escalated alcohol self-administration and (ii) reductions in DYN/KOR dysregulation that restores normal dose–responsivity to the system. The first process could be applicable to both currently identified explanations for the long-term effects of nor-BNI (ie, through stimulation of c-Jun N-terminal kinase phosphorylation (Bruchas et al, 2007; Melief et al, 2011) or as a function of nor-BNI accumulation in the brain (Patkar et al, 2013)).
Methodologically notable are the present results showing that protracted escalated alcohol self-administration was observed following an extended duration (~16 weeks) of alcohol vapor exposure. Previous research has shown that artificially sweetened alcohol intake remains elevated following 7, but not 4, weeks of intermittent alcohol vapor exposure (Rimondini et al, 2003), which could explain situations in which intermittent alcohol vapor exposure produced escalated alcohol self-administration during acute withdrawal that did not persist into protracted abstinence. Nevertheless, long-term alcohol vapor exposure induced protracted escalated self-administration of non-adulterated 10% (w/v) alcohol consumption which supports the construct and predictive validity of intermittent alcohol vapor exposure for dependence induction and pharmacological evaluation of putative therapeutics to treat alcohol dependence.
The CeA is a critical component of a functionally interconnected network termed the ‘extended amygdala’ (comprised of the CeA, accumbens shell and bed nucleus of the stria terminalis (Alheid and Heimer, 1988)); with alterations in CeA function influencing a broad range of behaviors that encompass emotional/affective, conditional learning, motivation, and decision-making domains (Koob and Volkow, 2010). Neuroadaptations within the CeA are implicated in the pathophysiology of various neuropsychiatric disorders (Drevets, 2003) induced by chronic alcohol consumption. As a major output region of the amygdala, the CeA has been implicated as a substrate of negative emotional states accompanying chronic exposure to drugs of abuse (eg, alcohol and cocaine) and withdrawal-induced phenotypes (eg, escalated self-administration and increased anxiety) that are attenuated by intra-CeA KOR antagonism (Kallupi et al, 2013; Kissler et al, 2014). The capsular region of the CeA, also termed the ‘nociceptive’ amygdala, is a region of the CeA that has extensive connectivity with midbrain and brainstem nuclei, such as the periaqueductal gray. Reciprocal projections between the CeA and periaqueductal gray lay the foundation for a ‘FEAR’ circuit designed to deter organisms from evolutionarily disadvantageous situations (Wright and Panksepp, 2011). Ascending projections from the CeA innervate nuclei important for signaling biologically relevant information to promote survival (Berthoud, 2002), such as the ventral striatum, among others. The dense projection from the CeA to the bed nucleus of the stria terminalis, in concert with the ventral striatum, integrates emotional and motivational information and is recruited in dependence to promote excessive alcohol seeking and consumption (Koob, 2009), and KORs have been shown to modulate the function of all primary extended amygdala nuclei (Lindholm et al, 2007; Li et al, 2012; Kallupi et al, 2013). From a functional circuitry-based perspective, an important question for future research to assess would be the net effect of nor-BNI on CeA activity and output to different cortical, limbic, and brainstem nuclei.
Long-term changes in affective states following chronic exposure to drugs of abuse, including alcohol, could result from neuroadaptations within the extended amygdala and its rich connectivity (Koob and Le Moal, 2008). KORs pre-synaptically modulate several neurotransmitter systems in the extended amygdala that include dopaminergic inputs to the amygdala and accumbens; GABAergic inputs to the accumbens, amygdala, and bed nucleus of the stria terminalis; serotonergic inputs to the accumbens; and glutamatergic inputs to the accumbens (for review, see Crowley and Kash, 2015). Such modulation not only applies to drug and alcohol abuse-related dysregulation, but has considerable implications for the etiology and treatment of numerous neuropsychiatric disorders (Schwarzer, 2009; Sirohi et al, 2012). Therefore, dysregulation of the DYN/KOR system in the extended amygdala could contribute to escalated alcohol self-administration and the emergence of negative affective states during withdrawal.
In summary, site-specific KOR antagonism in the CeA rescued escalated alcohol self-administration during acute withdrawal and protracted abstinence independent of any effects on physiological withdrawal symptoms. Collectively, the present results address a significant question related to acute-withdrawal KOR antagonist efficacy and, importantly, identify that dysregulated motivation for alcohol during protracted abstinence is sensitive to intra-CeA KOR antagonism. As such, the present findings show that targeting KORs in the extended amygdala can ameliorate maladaptive symptoms of alcohol dependence and withdrawal that persist beyond acute withdrawal into protracted abstinence and are highly informative about the therapeutic efficacy of KOR antagonists. Indeed, the ability of KOR blockade to rescue one of the most persistent and deleterious withdrawal symptoms (ie, excessive motivation for alcohol) will aid in the discovery of novel therapeutics for alcohol use disorders.
Funding and Disclosure
The authors declare no conflict of interest.
References
Alheid GF, Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1–39.
Bazov I, Kononenko O, Watanabe H, Kuntic V, Sarkisyan D, Taqi MM et al (2013). The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict Biol 18: 161–169.
Berger AL, Williams AM, McGinnis MM, Walker BM (2013). Affective cue-induced escalation of alcohol self-administration and increased 22-kHz ultrasonic vocalizations during alcohol withdrawal: role of kappa-opioid receptors. Neuropsychopharmacology 38: 647–654.
Berthoud HR (2002). Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26: 393–428.
Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH (1994). Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 115: 311–319.
Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S et al (2007). Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem 282: 29803–29811.
Charney DA, Zikos E, Gill KJ (2010). Early recovery from alcohol dependence: factors that promote or impede abstinence. J Subst Abuse Treat 38: 42–50.
Crowley NA, Kash TL (2015). Kappa opioid receptor signaling in the brain: circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 62: 51–60.
De Soto CB, O'Donnell WE, De Soto JL (1989). Long-term recovery in alcoholics. Alcohol Clin Exp Res 13: 693–697.
Deehan GA Jr, McKinzie DL, Carroll FI, McBride WJ, Rodd ZA (2012). The long-lasting effects of JDTic, a kappa opioid receptor antagonist, on the expression of ethanol-seeking behavior and the relapse drinking of female alcohol-preferring (P) rats. Pharmacol Biochem Behav 101: 581–587.
Drevets WC (2003). Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci 985: 420–444.
Elholm B, Larsen K, Hornnes N, Zierau F, Becker U (2011). Alcohol withdrawal syndrome: symptom-triggered versus fixed-schedule treatment in an outpatient setting. Alcohol Alcohol 46: 318–323.
Hall W, Zador D (1997). The alcohol withdrawal syndrome. Lancet 349: 1897–1900.
Hyytia P (1993). Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav 45: 697–701.
Kallupi M, Wee S, Edwards S, Whitfield TW Jr ., Oleata CS, Luu G et al (2013). Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biol Psychiatry 74: 520–528.
Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG et al (2014). The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry 75: 774–782.
Koob GF (2009). Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42: S32–S41.
Koob GF, Le Moal M (2008). Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363: 3113–3123.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28: 407–414.
Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB et al (2012). Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry 71: 725–732.
Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J (2007). Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav 92: 167–171.
Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr . et al (2003). Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305: 323–330.
Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1987). Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7: 2445–2464.
Markou A, Kosten TR, Koob GF (1998). Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18: 135–174.
Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA Jr ., Cohen BM et al (2011). Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol 80: 920–929.
National Research Council, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, National Academies Press (2011) Guide for the Care and Use of Laboratory Animals. National Academies Press: Washington, D.C..
Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011). kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61: 35–42.
Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K et al (2013). Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J Pharmacol Exp Ther 346: 545–554.
Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. Elsevier, Academic Press: San Diego.
Picker MJ, Mathewson C, Allen RM (1996). Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol 7: 495–504.
Rimondini R, Sommer W, Heilig M (2003). A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol 64: 445–449.
Roelofs SM (1985). Hyperventilation, anxiety, craving for alcohol: a subacute alcohol withdrawal syndrome. Alcohol 2: 501–505.
Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL (1997). Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry 154: 948–957.
Schwarzer C (2009). 30 years of dynorphins—new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 123: 353–370.
Sirohi S, Bakalkin G, Walker BM (2012). Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci 5: 95.
Spanagel R (2009). Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89: 649–705.
Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X (2014). Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 11: E109.
Valdez GR, Harshberger E (2012). Kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav 102: 44–47.
Walker BM (2012). Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol 46: 339–348.
Walker BM, Koob GF (2007). The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res 31: 11–18.
Walker BM, Koob GF (2008). Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33: 643–652.
Walker BM, Valdez GR, McLaughlin JP, Bakalkin G (2012). Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol 46: 359–370.
Walker BM, Zorrilla EP, Koob GF (2011). Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol 16: 116–119.
Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE et al (2012). The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 223: 75–88.
Wright JS, Panksepp J (2011). Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci Biobehav Rev 35: 1902–1915.
Zhao Y, Weiss F, Zorrilla EP (2007). Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res 31: 1505–1515.
Acknowledgements
Support for this research was provided in part by R01AA020394 from the National Institute on Alcohol Abuse and Alcoholism and grants from the WSU Alcohol and Drug Abuse Research Program awarded to BMW according to the State of Washington Initiative Measure No. 17. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington. BMW is a consultant for H. Lundbeck A/S (Copenhagen, Denmark) and has received honoraria for speaking at Lundbeck-hosted scientific meetings.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kissler, J., Walker, B. Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala κ-Opioid Receptors. Neuropsychopharmacol 41, 560–567 (2016). https://doi.org/10.1038/npp.2015.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.183
This article is cited by
-
Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice
Neuropsychopharmacology (2019)
-
Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys
Neuropsychopharmacology (2019)
-
Role of κ-Opioid Receptors in the Bed Nucleus of Stria Terminalis in Reinstatement of Alcohol Seeking
Neuropsychopharmacology (2018)
-
Opioid receptors: drivers to addiction?
Nature Reviews Neuroscience (2018)