Abstract
Ecstasy (±3,4-methylenedioxymethamphetamine, MDMA) is a popular recreational drug with known serotonergic neurotoxicity. Its long-term effects on dopaminergic function are less certain. Studying the long-term effects of ecstasy is often confounded by concomitant polydrug use and the short duration of abstinence. We used 18F-dopa positron emission tomography (PET) to investigate the long-term effects of ecstasy on nigrostriatal dopaminergic function in a group of male ex-recreational users of ecstasy who had been abstinent for a mean of 3.22 years. We studied 14 ex-ecstasy users (EEs), 14 polydrug-using controls (PCs) (matched to the ex-users for other recreational drug use), and 12 drug-naive controls (DCs). Each participant underwent one 18F-dopa PET, cognitive assessments, and hair and urinary analyses to corroborate drug-use history. The putamen 18F-dopa uptake of EEs was 9% higher than that of DCs (p=0.021). The putamen uptake rate of PCs fell between the other two groups, suggesting that the hyperdopaminergic state in EEs may be due to the combined effects of ecstasy and polydrug use. There was no relationship between the amount of ecstasy used and striatal 18F-dopa uptake. Increased putaminal 18F-dopa uptake in EEs after an abstinence of >3 years (mean) suggests that the effects are long lasting. Our findings suggest potential long-term effects of ecstasy use, in conjunction with other recreational drugs, on nigrostriatal dopaminergic functions. Further longitudinal studies are required to elucidate the significance of these findings as they may have important public health implications.
Similar content being viewed by others
INTRODUCTION
Ecstasy (MDMA, ±3,4-methylenedioxymethamphetamine) is an amphetamine derivative, which is the main psychoactive compound in ‘ecstasy’ tablets. Ecstasy is a popular recreational drug and is frequently taken in multiple doses in nightclubs and parties. In the United Kingdom, the prevalence of ecstasy use in the 15–34 years age group is 13.6% (EMCDDA, 2005). Neurotoxicity of MDMA on the serotonergic system, as defined by a reduction in neuronal or neurochemical markers, has been demonstrated in both animals and humans (Green et al, 2003), although this may be partly reversible after a prolonged period of abstinence (for review, see Cowan (2007) and Reneman et al (2006)). The effects of MDMA on dopaminergic neurons are less certain. Acutely, MDMA releases dopamine from the dopaminergic terminals (Colado et al, 2004). In animals, with the exception of mice and, under certain conditions, rats (Commins et al, 1987; Yuan et al, 2002), MDMA has not been found to cause dopamine neurotoxicity (Colado et al, 2004; Logan et al, 1988)—unlike amphetamine (Melega et al, 1997) or methamphetamine (Wilson et al, 1996). In humans, some studies have suggested the chronic effects of MDMA usage on dopaminergic neurons. One study showed that 3 weeks after discontinuation of MDMA, the growth hormone response to the dopamine agonist bromocriptine was significantly attenuated in ecstasy users compared with controls, possibly reflecting reduced dopamine D2 receptor sensitivity in the hypothalamus (Gerra et al, 2002). There have also been several reports suggesting an association between MDMA usage and subsequent development of parkinsonism (Kuniyoshi and Jankovic, 2003; Mintzer et al, 1999), but no definite causal link has been established.
The interpretation of many studies involving ecstasy users can be confounded by concomitant polydrug use, given that most ecstasy users have also taken other recreational drugs (Gouzoulis-Mayfrank and Daumann, 2006). Previous neuroimaging studies investigating the effects of ecstasy on the dopaminergic system have produced heterogeneous results. The relatively short period of abstinence from ecstasy in participants included in these studies also cannot address long-term toxicity. Using 123I--CIT single-photon emission computed tomography, one study reported no difference in striatal dopamine transporter (DAT) uptake between a group of current MDMA users and polydrug-using controls (PCs) (Semple et al, 1999). Another study showed an increase in striatal DAT binding in ecstasy users who had been abstinent for at least 3 weeks compared with controls and ecstasy users who also used amphetamine (Reneman et al, 2002). Finally, one group used 11C-WIN35428 positron emission tomography (PET) and did not find any difference in striatal DAT binding between MDMA users who had been abstinent for a mean of 2.75 months and a group of unmatched polydrug users (McCann et al, 2008).
18F-dopa PET provides an in vivo marker of presynaptic dopaminergic terminal function (Brooks, 2003). Using 18F-dopa PET, we investigated the long-term effects of ecstasy exposure on nigrostriatal dopaminergic function in ex-ecstasy users (EEs) who had abstained from this drug for at least a year. The aspects of mood and cognition associated with dopaminergic function or with impairment after ecstasy use were also assessed. We included a group of PCs to minimize the possible confounding effects of exposure to other recreational drugs.
MATERIALS AND METHODS
Design and Participants
We recruited three groups of healthy participants: (1) EEs who had taken ecstasy on at least 25 occasions but had not taken any for the past 12 months, although they could continue to use other recreational drugs; (2) PCs who were matched to EEs in their recreational drug use but had never taken ecstasy; and (3) drug-naive controls (DCs) who had never taken any recreational drugs except alcohol. The 25 occasions of ecstasy use were an arbitrary cutoff to exclude very light ex-users from this study. Participants were recruited through magazine advertisements and word of mouth. They were not informed of the inclusion criteria beforehand, and they were reimbursed travel expenses and £50 for their time. All three groups were matched for age, premorbid IQ, level of education, and for the drug-using groups, history of drug use apart from ecstasy.
Inclusion criteria for all groups were that participants be male, aged 25–50 years, not take prescribed psychotropic medication or receive psychological treatment, no current or history of drug addiction, not be depressed as determined by the Structured Clinical Interview for DSM-IV (First et al, 1997), have a score of <18 on the Beck Depression Inventory, have a score of <55 on the Spielberger Trait Anxiety Scale, have no significant medical illness or serious head injuries in the past, and no history of schizophrenia or parkinsonism among first-degree relatives. Participants were also required not to drink >3 Units of alcohol in the 24 h before testing and not to use any recreational drugs for at least 3 days before testing (for the drug-using groups). Only male volunteers above the age of 25 years were included because the local ethics committee has imposed restrictions on exposing young adults and healthy women of childbearing age to ionizing radiation. All participants underwent a neurological examination to detect any signs of parkinsonism. Participants in this study were part of a larger cohort of volunteers who participated in a related neuropsychological study (Hoshi et al, 2007), in which a separate subgroup also underwent 11C-DASB PET to assess serotonin transporter status (Selvaraj et al, 2009).
PET scanning and neuropsychological testing were performed on the same day. Each participant also provided urine and hair samples on the same day, which were analyzed to corroborate their account of recent and longer-term history of drug use, respectively. The hair and urine samples were tested using enzyme-multiplied immunoassay to detect the presence of amphetamines, cocaine, and methamphetamines groups of drugs, whereas the urine samples were tested additionally for opiates, benzodiazepines, barbiturates, and cannabis. If the initial screen was positive for amphetamines, cocaine, methamphetamines, or opiates, the sample was further analyzed by gas/liquid chromatography-mass spectrometry to determine the actual substance present. Most recreational drugs persist in the urine for 48–72 h after ingestion apart from cannabis, which could be detected for 2–3 weeks after use. Every 1 cm of hair length approximates to a 1-month window of detection. The duration tested is limited by the length of hair available for analysis (Pragst and Balikova, 2006). Hair analysis was performed by TrichoTech (Cardiff, UK). If the urine or hair analysis showed that participants no longer fulfilled the inclusion criteria, then they would be excluded from further analysis.
The study was approved by the local research ethics committee, and permission to administer 18F-dopa was granted by the Administration of Radioactive Substances Advisory Committee of the United Kingdom. All participants gave written informed consent to participate in this study in accordance with the Declaration of Helsinki.
PET Scanning
All participants underwent one 18F-dopa PET. PET was performed in the morning and participants were asked to have a light breakfast at least 4 h before imaging and to avoid caffeine for at least 12 h. Participants arrived at our PET unit at least 2 h before the scan, so that they could settle down in a controlled environment. They were orally administered 150 mg of carbidopa and 400 mg of entacapone 1 h before injection of 18F-dopa to block the activity of peripheral aromatic amino-acid decarboxylase and catechol-O-methyl transferase, respectively. PET was performed using an ECAT EXACT HR++ (Knoxville, TN) camera. The camera has a transaxial spatial resolution of 4.8±0.2 mm and an axial resolution of 5.6±0.5 mm after image reconstruction. A 5-min transmission scan was performed before injection of 18F-dopa to correct for tissue attenuation of 511 keV γ-radiation. In all, 114.5±4.7 (mean ± SD) MBq of 18F-dopa was injected intravenously over 30 s, and the dynamic emission data were acquired as 26 time frames over 95 min.
Image Analysis
The investigator (YFT) analyzing the scans was blinded to the drug-use history of participants.
18F-dopa dynamic images were corrected for between-frame head movements during PET scans by applying a frame-by-frame realignment paradigm (Lang et al, 2006; Montgomery et al, 2006). In brief, nonattenuation-corrected dynamic images were first denoized using a Battle-Lemarie wavelet (Turkheimer et al, 1999). Frames 4–26 of dynamic scans were coregistered to frame 3 (the first frame with a high signal-to-noise ratio), and transformation matrices were applied to the corresponding frames of the attenuation-corrected dynamic images.
18F-dopa images were analyzed as described previously (Whone et al, 2004). Individual parametric images of 18F-dopa influx rate constant (Ki maps) were created using a standard multiple-time graphical approach (Patlak and Blasberg, 1985), with an occipital reference input function. An ‘ADD’ image of integrated 18F-dopa signal from 30 to 90 min was also created for each participant. The ADD images were then spatially normalized to an in-house 18F-dopa template in a standard stereotactic (MNI) space using the statistical parametric mapping (SPM) software (Wellcome Functional Imaging Laboratory, London). The transformation matrices were then applied to the corresponding Ki maps. A standard region-of-interest object map that outlined the putamen, heads of caudate nucleus, and ventral striatum was defined on the 18F-dopa template with magnetic resonance imaging guidance (Whone et al, 2004). This object map was then applied to each normalized parametric Ki map to measure individual Ki values. For each subject, left- and right-sided Ki values were averaged.
Cognitive and Mood Assessments
The participants in this study form a part of a larger cognitive study, and the cognitive and mood assessments used have been described elsewhere (Hoshi et al, 2007). They included, among others, tests which are known to tap dopaminergic and serotonergic functions: immediate and delayed prose recall (Rivermead Behavioural Memory Tests; Wilson et al, 1985), the Buschke Selective Reminding Task (BSRT; Buschke and Fuld, 1974), Go/No-Go task (Mesulam, 1985), Rapid Visual Information Processing (Wesnes and Warburton, 1983), the Serial Sevens task (Hayman, 1942), semantic and phonemic verbal fluency, the Trail Making Test (Reitan, 1959), CANTAB spatial working memory (Owen et al, 1990), CANTAB Stockings of Cambridge (Owen et al, 1995), and Gibson's spiral maze (Gibson, 1977). Impulsivity was assessed using the Barratt Impulsiveness Scale (Barratt and Patton, 1983), and aggression was measured using the Aggression Questionnaire (Buss and Perry, 1992).
Drug-Use History
A semi-structured interview was carried out on all participants to ascertain their levels of drug usage. For each drug, a lifetime estimate of amount used was obtained using the formula: (number of days used per month) × 12 × (number of years of regular use) × (dosage per session).
Statistical Analysis
Statistical analysis was performed using SPSS v16 (SPSS Software, Chicago, IL). The Kolmogorov–Smirnov test confirmed the normal distribution of drug-use data. The majority of group differences were assessed using one-way ANOVA. The prose recall task was analyzed using repeated-measures ANOVA. Post hoc comparisons were performed with a Bonferroni correction and the significance level was set at p=0.05.χ2-Tests were used to compare nonparametric data. Pearson's correlations were used to explore the relationship between PET data, drug use, and cognitive performance. Owing to the number of correlations performed, α=0.01 was adopted to minimize the probability of type I errors.
RESULTS
Demographic Details and Drug-Use History
A total of 46 participants were tested: 17 EEs, 16 PCs, and 13 DCs. Several participants were eventually excluded from the analysis as they were found not to fulfil the inclusion criteria: three EEs (two tested positive for cocaine in urine analysis indicating recent cocaine use, one reported the use of ecstasy 4 months before testing), two PCs (one due to scan failure and one tested positive for MDMA in hair analysis), and one DC (tested positive for MDMA in hair analysis). Thus, the final analysis included 14 EEs, 14 PCs, and 12 DCs. One PC was diagnosed with ‘depressed mood’ by his family doctor more than a year before his PET study and was subjected to a trial of a selective serotonin reuptake inhibitor for 2 weeks. He decided to discontinue medication after that as he believed that he was much better. There was no other history of psychiatry disorder or history of use of psychotropic drugs among the participants.
The demographic details of the participants are listed in Table 1. There were no group differences in age, premorbid IQ (Spot the Word), depression, anxiety, aggression, and impulsivity (Table 1), or groups in level of education attained and current employment status (not listed). All participants underwent a normal neurological examination. EEs have on average been using ecstasy for 4.38 years but have abstained from it for 3.22 years (Table 2). There was no significant difference in the drug-use pattern of EEs and PCs (Table 3). A group difference was found in units of alcohol consumed per week (F2, 39=4.479, p=0.018), with PCs reported drinking more than DCs (p=0.02, corrected). Three participants tested positive for cocaine on hair analysis: one EE (within the last 3 months based on hair analysis, self-reported last use 5 days ago) and two PCs (one PC within the last 3 months, self-reported last use 30 days ago; the second PC within the last 3.5 months, self-reported last use 5 days ago). The remaining participants tested negative on hair analysis. Excluding these three participants, the average length of hair tested was ∼3 cm in all three groups, equivalent to 3 months being free from amphetamines, cocaine, and methamphetamines groups of drugs (Table 3). Three participants also tested positive for cannabis in urine: one EE (the same participant who also tested positive for cocaine on hair analysis) and two PCs.
Striatal 18F-Dopa Uptake
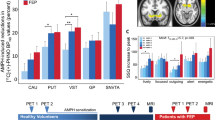
One-way ANOVA detected a significant group difference in the putamen 18F-dopa uptake ((F2, 39=4.108, p=0.024); Table 4 and Figure 1). Post hoc comparison revealed that EEs had 9% higher putamen 18F-dopa uptake than did DCs (p=0.021, corrected). The putamen 18F-dopa uptake rate of PCs fell between that of EEs and DCs, but there were no significant group differences. There were no significant group differences in 18F-dopa uptake in the ventral striatum and caudate nucleus, although these were 6 and 5% higher, respectively, in EEs than in DCs. Exclusion of the five participants who tested positive for cocaine and cannabis on hair and urine analyses had minimal impact on the results (putamen 18F-dopa uptake (F2, 34=3.969, p=0.029); EE>DC, p=0.025, corrected).
Mean 18F-dopa uptake in the putamen of ex-ecstasy users, polydrug-using controls, and drug-naive controls. Error bars indicate SD. Significant comparison: *Ex-ecstasy users>drug-naive controls (p=0.021, corrected).
To further ensure that the differences seen in the putamen 18F-dopa uptake were not due to the influences of other recreational drugs which could potentially affect dopaminergic activity, we performed an ANCOVA with putamen 18F-dopa uptake as the dependent variable, participants' drug-use group as the fixed factor, and lifetime estimated amounts of amphetamine, cocaine, and cannabis as covariates. We also included the amount of alcohol consumed as a covariate as there were group differences in its consumption. ANCOVA confirmed a significant effect of group on putamen 18F-dopa uptake (F2, 33=4.116, p=0.025), with EEs having higher values than DCs (p=0.024, corrected). The effects of other recreational drugs on putamen 18F-dopa uptake were not significant.
Neuropsychological Data
Data for two participants (one EE and one DC) on the RVIP and Go/No-Go were lost due to computer failure.
A significant group difference was observed on the first trial of the BSRT (F2, 39=5.85, p=0.006). Post hoc tests revealed that both EEs (p=0.04) and PCs (p=0.01) recalled fewer words than did DCs. Analysis of the Go/No-Go task (Table 5) revealed a significant group difference on the number of hits (χ2=7.57, df=2, p=0.023), with PCs making significantly fewer hits than EEs (p=0.008). A group difference was also found in the number of false alarms (F2, 37=6.16, p=0.005): PCs made more false alarms than did both EEs (p=0.01) and DCs (p=0.02). A group difference was observed on reaction times to hits (F2, 37=0.395, p=0.028). PCs reacted faster to hits than did EEs (p=0.03). There were no significant group differences in other assessments.
Correlations
EEs group
18F-dopa uptake in the putamen correlated negatively with the total number of false alarms on the Go/No-Go task (r=−0.815, p=0.001) and correlated positively with reaction time to hits (r=0.761, p=0.003). There was no correlation between striatal 18F-dopa uptake and the following: lifetime amount of ecstasy or other recreational drugs, time since last use of ecstasy, and Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE) scores (see below).
Assessment of Schizotypy
The striatal hyperdopaminergic state found in EEs was not predicted a priori. The main disorder in the literature that demonstrates a consistent association with a hyperdopaminergic state is psychosis, usually in the context of schizophrenia (Hietala et al, 1995; Howes et al, 2007). Therefore, we performed a post hoc assessment using the O-LIFE short scale (Mason et al, 2005) to measure participants' schizotypy or psychosis-proneness as none of our participants had frank psychotic symptoms. O-LIFE consists of four subscales, namely Unusual Experiences, Cognitive Disorganisation, Introvertive Anhedonia, and Impulsive Nonconformity (Table 6). It was administered within 3 months of completion of their PET scans. The rate of response and O-LIFE scores are listed in Table 6. There were no group differences in the response rates or O-LIFE scores. The relatively poor response rate was probably due to the post hoc nature of the questionnaire with associated dropouts.
DISCUSSION
This is the first study looking into the long-term effects of ecstasy exposure, in the setting of polydrug use, on the nigrostriatal dopaminergic system after a prolonged period of abstinence. We detected an increase in the putamen 18F-dopa uptake in a group of male EEs, who had abstained for >3 years (mean), compared with DCs. The putamen 18F-dopa uptake rate of PCs lay between that of EEs and DCs, suggesting that the difference seen between the latter two groups may be due to the combined effects of ecstasy and polydrug use rather than ecstasy alone. The lack of correlation between striatal 18F-dopa uptake and ecstasy use also suggests that polydrug use could be an important factor. We have not demonstrated any evidence of ecstasy-induced nigrostriatal dopaminergic neurotoxicity.
As mentioned in the ‘Introduction,’ section, previous neuroimaging studies investigating the effects of ecstasy on nigrostriatal dopaminergic functions produced rather mixed results. There were significant methodological differences, including periods of abstinence and control groups chosen, with our study which made direct comparisons difficult. None of the studies mentioned used hair analyses to corroborate the drug-use history of their participants. Semple et al (1999) studied current ecstasy users, not ex-users, and they did not include any DCs. McCann et al (2008) did not detect any difference in DAT binding in ecstasy users who had been abstinent for 2.75 months (mean) and a group of controls with low-level polydrug use; but they did not include a DC group which may partially explain the lack of significant difference in DAT binding. Reneman et al (2002) reported significantly higher striatal DAT binding in a group of ecstasy users who had never used amphetamine and who had been abstinent from ecstasy for a mean 3.4 months, compared with a group of ecstasy users who had also used amphetamine and a group of controls who had never used these two drugs. The conclusion of the study focused on the ‘lower’ DAT binding with amphetamine use, which in fact was not different from controls, rather than the increase in DAT binding with ecstasy use. The authors proposed that amphetamine, a probable dopamine neurotoxin, in conjunction with ecstasy might be responsible for the lower DAT binding compared with ecstasy users naive to amphetamine. Of the three studies quoted above, the latter study had a more similar set-up with our study, with relatively drug-free control groups. Taken together with their findings, our results are compatible with the presence of a striatal hyperdopaminergic state in EEs and its persistence after an abstinence from ecstasy for >3 years mean suggests that the effects maybe long lasting.
A postmortem study found increased homovanillic acid, a dopamine metabolite, in the putamen of the single MDMA chronic user studied compared with DCs (Kish et al, 2000), indicating possible increased dopamine turnover. Other studies have also shown increased cerebrospinal fluid and plasma homovanillic acid in patients with psychosis (Maas et al, 1997; Pickar et al, 1986; Ramirez-Bermudez et al, 2008), a condition linked to the hyperdopaminergic state. An increase in striatal 18F-dopa uptake has been reported in neuropsychiatric conditions particularly in schizophrenia and psychosis—including those with prodromal psychotic symptoms (Howes et al, 2007, 2009); whereas less consistent findings have been observed in Tourette's syndrome and attentional deficit hyperactivity disorder (Nikolaus et al, 2009), which are mainly disorders of childhood onset. The magnitude of increase in putamen 18F-dopa uptake in EEs compared with DCs in our study is similar to that seen in at-risk mental state subjects who have prodromal symptoms of schizophrenia (Howes et al, 2009). The O-LIFE questionnaire in our study did not reveal any significant differences in schizotypy among our participants, but the results are limited by the variable response rate, and the retrospective and cross-sectional nature of our survey. Chronic ecstasy use may lead to multiple long-term neuropsychiatric consequences, including psychosis (McGuire et al, 1994; Montoya et al, 2002; Winstock, 1991). One study looked at substance abuse in patients presenting with first-episode psychosis and found that age at first use of cannabis, cocaine, ecstasy, and amphetamine was independently associated with age at first psychotic symptom (Barnett et al, 2007).
The underlying molecular mechanisms for ecstasy-related hyperdopaminergic state are still unclear, but our findings suggest they may be related to synergistic or interactive effects with other recreational drugs. The increased striatal 18F-dopa uptake and DAT binding are consistent with increased dopamine turnover. This may be a reaction to chronic exposure to a dopamine-depleting agent or may reflect long-term sensitization of the nigrostriatal dopaminergic system to MDMA, in which repeated exposure to a stimulant drug resulted in heightened dopamine release after re-exposure, as demonstrated in vivo with amphetamine 1 year after limited exposure to the substance (Boileau et al, 2006). Acutely, MDMA increases DOPA accumulation in the striatum, which is blocked by the 5-HT2 antagonist ketanserin, suggesting that MDMA activates the nigrostriatal dopaminergic pathways through 5-HT2 receptors (Nash et al, 1990). It is uncertain whether this effect persists after repeated dosing or prolonged abstinence, but this mechanism could offer a potential explanation for our findings.
An alternative, although speculative, explanation for our findings is that there are preexisting differences in the nigrostriatal dopaminergic system between ecstasy and nonecstasy users, ie, the hyperdopaminergic state leads to a desire to use stimulants, such as ecstasy. We do not have baseline or predrug-use data on these participants. However, a longitudinal multi-modality neuroimaging study did not reveal any difference in the baseline neuroimaging data between subjects who remained ecstasy naive vs those who started using ecstasy at follow-up, although dopaminergic function was not measured (de Win et al, 2008).
Cognitive assessments revealed minor differences across groups. Both drug-using groups were impaired on the BSRT, indicating they had a shorter immediate memory span. The lack of major cognitive impairment in EEs is in line with other published studies (Hoshi et al, 2007; McCann et al, 2008). These cognitive tests probably have lower sensitivity than PET in detecting neurochemical abnormalities in the drug users, as previous studies have demonstrated reduced serotonin transporters in ecstasy users but without concomitant changes in their cognitive performances (McCann et al, 2008). Higher putamen 18F-dopa uptake in EEs correlated with fewer false alarms, but a slower reaction time to hits on a choice reaction time task. The significance of these correlations is unclear and it needs to be interpreted with caution as there were no differences between EEs and DCs in these parameters. We excluded participants with significant anxiety or depression, which can often be seen in chronic drug users. This may also explain the lack of group differences in the personality traits and psychological data in Table 1.
There are several potential limitations of our study. As with all studies involving recreational drug use, we have to rely on participants' subjective recall of their drug-use history. We attempted to corroborate that as far as possible with urine and hair analyses but inevitably recall biases or errors could occur. The small number of participants tested positive for cannabis on urine analysis and cocaine on hair analysis indicated that the confounding effects from recent use of other drugs were likely to be small. Excluding these participants from analysis or incorporating other drug use as covariates in the ANCOVA analysis did not alter our study results. The average duration of 3 months being free from amphetamines, cocaine, and methamphetamines groups of drugs as shown on hair analysis also implied that the impact from recent ingestion of these drugs on the dopaminergic system was likely to be small. Nevertheless, we were unable to test for the longer history of drug use, especially with regard to ecstasy to verify that EEs had indeed abstained from the drug for more than a year. Although we tried to minimize the confounding effects of polydrug use by matching EE and PC groups, in reality it was difficult to do so exactly, especially when we also had to rely on subjective recalls of the participants. One further limitation is that the purity of recreational drugs taken by the participants in our study cannot be ascertained. Our study included male participants only, and hence the results may not be generalized to female EEs. One study has reported that women might be more susceptible than men to MDMA-induced alterations of the serotonergic system (Reneman et al, 2001). EEs showed a modest 9% increase in the putamen 18F-dopa uptake compared with DCs. Although this is significantly greater than the striatal 18F-dopa uptake test–retest variability of ∼5% (Egerton et al, 2010) and of a similar magnitude seen in at-risk mental state subjects with prodromal symptoms of schizophrenia (Howes et al, 2009), it would be prudent to repeat this in a larger-scale study. Finally, we studied participants with a history of recreational drug use only. Therefore, our results may not be applicable to those with drug addiction and heavy use.
The long-term sequelae of our findings are as yet uncertain, and follow-up of the EEs would be important to evaluate any development of psychopathology or further changes in dopaminergic function. Nevertheless, our study highlights potential long-term adverse effects from recreational use of a very popular illicit substance, in the setting of polydrug use, with consequent public health implications. Larger-scale prospective studies (de Win et al, 2008) would be able to confirm and to further delineate the effects of ecstasy on human nigrostriatal dopaminergic system, while avoiding difficulties and limitations frequently encountered with cross-sectional drug studies as outlined above.
References
Barnett JH, Werners U, Secher SM, Hill KE, Brazil R, Masson K et al (2007). Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry 190: 515–520.
Barratt E, Patton JH (1983). Impulsivity: cognitive, behavioural and psychophysiological correlates. In: Zuckerman M (ed). Biological Basis of Sensation Feeling, Impulsivity and Anxiety. Erlbaum: Hillsdale, NJ.
Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M et al (2006). Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 63: 1386–1395.
Brooks DJ (2003). Imaging end points for monitoring neuroprotection in Parkinson's disease. Ann Neurol 53: S110–S118.
Buschke H, Fuld PA (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24: 1019–1025.
Buss AH, Perry M (1992). The aggression questionnaire. J Pers Soc Psychol 63: 452–459.
Colado MI, O'Shea E, Green AR (2004). Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 173: 249–263.
Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS (1987). Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 241: 338–345.
Cowan RL (2007). Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 189: 539–556.
de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C et al (2008). Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain 131: 2936–2945.
Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD (2010). The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage 50: 524–531.
EMCDDA (2005). Annual Report 2005: The State of the Drugs Problem in Europe. EMCDDA: Luxembourg.
First MB, Spitzer RL, Gibbon M, Williams JB (1997). Structured Clinical Interview for DSM–IV Axis I Disorders. American Psychiatric Publishing: Washington, DC.
Gerra G, Zaimovic A, Moi G, Giusti F, Gardini S, Delsignore R et al (2002). Effects of (+/−) 3,4-methylene-dioxymethamphetamine (ecstasy) on dopamine system function in humans. Behav Brain Res 134: 403–410.
Gibson H (1977). Manual of the Gibson Spiral Maze. Hodder: London.
Gouzoulis-Mayfrank E, Daumann J (2006). The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J Psychopharmacol 20: 188–193.
Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI (2003). The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). Pharmacol Rev 55: 463–508.
Hayman MAX (1942). Two minute clinical test for measurement of intellectual impairment in psychiatric disorders. Arch Neurol Psychiatry 47: 454–464.
Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M . et al (1995). Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet 346: 1130–1131.
Hoshi R, Mullins K, Boundy C, Brignell C, Piccini P, Curran HV (2007). Neurocognitive function in current and ex-users of ecstasy in comparison to both matched polydrug-using controls and drug-naive controls. Psychopharmacology (Berl) 194: 371–379.
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK (2007). Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl 51: s13–s18.
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P . et al (2009). Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 66: 13–20.
Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS (2000). Striatal serotonin is depleted in brain of a human MDMA (ecstasy) user. Neurology 55: 294–296.
Kuniyoshi SM, Jankovic J (2003). MDMA and parkinsonism. N Engl J Med 349: 96–97.
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R et al (2006). Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59: 459–466.
Logan BJ, Laverty R, Sanderson WD, Yee YB (1988). Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur J Pharmacol 152: 227–234.
Maas JW, Bowden CL, Miller AL, Javors MA, Funderburg LG, Berman N et al (1997). Schizophrenia, psychosis, and cerebral spinal fluid homovanillic acid concentrations. Schizophr Bull 23: 147–154.
Mason O, Linney Y, Claridge G (2005). Short scales for measuring schizotypy. Schizophr Res 78: 293–296.
McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT et al (2008). Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine (‘ecstasy’) users: relationship to cognitive performance. Psychopharmacology (Berl) 200: 439–450.
McGuire PK, Cope H, Fahy TA (1994). Diversity of psychopathology associated with use of 3,4-methylenedioxymethamphetamine (‘ecstasy’). Br J Psychiatry 165: 391–395.
Melega WP, Raleigh MJ, Stout DB, Huang SC, Phelps ME (1997). Ethological and 6-[18F]fluoro-L-DOPA-PET profiles of long-term vulnerability to chronic amphetamine. Behav Brain Res 84: 259–268.
Mesulam MM (1985). Principles of Behavioral Neurology. F.A. Davis: Philadelphia, PA.
Mintzer S, Hickenbottom S, Gilman S (1999). Parkinsonism after taking ecstasy. N Engl J Med 340: 1443.
Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, Mustafovic S, Grasby PM (2006). Correction of head movement on PET studies: comparison of methods. J Nucl Med 47: 1936–1944.
Montoya AG, Sorrentino R, Lukas SE, Price BH (2002). Long-term neuropsychiatric consequences of ‘ecstasy’ (MDMA): a review. Harv Rev Psychiatry 10: 212–220.
Nash JF, Meltzer HY, Gudelsky GA (1990). Effect of 3,4-methylenedioxymethamphetamine on 3,4-dihydroxyphenylalanine accumulation in the striatum and nucleus accumbens. J Neurochem 54: 1062–1067.
Nikolaus S, Antke C, Muller HW (2009). In vivo imaging of synaptic function in the central nervous system: II. Mental and affective disorders. Behav Brain Res 204: 32–66.
Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28: 1021–1034.
Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW (1995). Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 33: 1–24.
Patlak CS, Blasberg RG (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590.
Pickar D, Labarca R, Doran AR, Wolkowitz OM, Roy A, Breier A et al (1986). Longitudinal measurement of plasma homovanillic acid levels in schizophrenic patients. Correlation with psychosis and response to neuroleptic treatment. Arch Gen Psychiatry 43: 669–676.
Pragst F, Balikova MA (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370: 17–49.
Ramirez-Bermudez J, Ruiz-Chow A, Perez-Neri I, Soto-Hernandez JL, Flores-Hernandez R, Nente F et al (2008). Cerebrospinal fluid homovanillic acid is correlated to psychotic features in neurological patients with delirium. Gen Hosp Psychiatry 30: 337–343.
Reitan RM (1959). A Manual for the Administration and Scoring of the Trail Making Test. Indiana University Press: Indianapolis, USA.
Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB et al (2001). Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet 358: 1864–1869.
Reneman L, Booij J, Lavalaye J, de Bruin K, Reitsma JB, Gunning B et al (2002). Use of amphetamine by recreational users of ecstasy (MDMA) is associated with reduced striatal dopamine transporter densities: a [123I]beta-CIT SPECT study–preliminary report. Psychopharmacology (Berl) 159: 335–340.
Reneman L, de Win MM, van den Brink W, Booij J, den Heeten GJ Neuroimaging findings with MDMA/ecstasy: technical aspects, conceptual issues and future prospects. J Psychopharmacol, (2006) 20: 164–175.
Selvaraj S, Hoshi R, Bhagwagar Z, Murthy NV, Hinz R, Cowen P . et al (2009). Brain serotonin transporter binding in former users of MDMA (‘ecstasy’). Br J Psychiatry 194: 355–359.
Semple DM, Ebmeier KP, Glabus MF, O'Carroll RE, Johnstone EC (1999). Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry 175: 63–69.
Turkheimer FE, Brett M, Visvikis D, Cunningham VJ (1999). Multiresolution analysis of emission tomography images in the wavelet domain. J Cereb Blood Flow Metab 19: 1189–1208.
Wesnes K, Warburton DM (1983). Effects of smoking on rapid information processing performance. Neuropsychobiology 9: 223–229.
Whone AL, Bailey DL, Remy P, Pavese N, Brooks DJ (2004). A technique for standardized central analysis of 6-(18)F-fluoro-L-DOPA PET data from a multicenter study. J Nucl Med 45: 1135–1145.
Wilson B, Cockburn J, Baddeley A (1985). The Rivermead Behavioural Memory Test. Thames Valley Text: Reading, UK.
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al (1996). Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2: 699–703.
Winstock AR (1991). Chronic paranoid psychosis after misuse of MDMA. BMJ 302: 1150–1151.
Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA (2002). Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J Neurochem 80: 960–969.
Acknowledgements
The authors thank Oliver Howes for his helpful comments on the paper. The work was supported by Parkinson's Disease Society (UK). RH was supported by a Medical Research Council studentship, and YFT was supported by a Wellcome Trust fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DJB serves or has served on scientific advisory boards for CeNes, Synosia, Genentech, GlaxoSmithKline, and Orion; receives funding for travel from TEVA; serves or has served as an editorial board member for the Journal of Neural Transmission, Brain, Movement Disorders, the Journal of Neurology, Neurosurgery and Psychiatry, and Synapse; has received speaker honoraria from GlaxoSmithKline and Orion Pharma; serves as the Head of Neurology for GE Healthcare; and receives research support from Alzheimer's Research Trust and the Medical Research Council Clinical Sciences Centre. PP serves as a member of The Research Advisory Panel for Parkinson's Disease Society (UK) and receives research support from Parkinson's Disease Society (UK) and the Michael J Fox Foundation (USA). The other authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Tai, Y., Hoshi, R., Brignell, C. et al. Persistent Nigrostriatal Dopaminergic Abnormalities in Ex-Users of MDMA (‘Ecstasy’): An 18F-Dopa PET Study. Neuropsychopharmacol 36, 735–743 (2011). https://doi.org/10.1038/npp.2010.201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.201
Keywords
This article is cited by
-
Neuroimaging of reward mechanisms in Gambling disorder: an integrative review
Molecular Psychiatry (2019)
-
Progression and Persistence of Neurotoxicity Induced by MDMA in Dopaminergic Regions of the Mouse Brain and Association with Noradrenergic, GABAergic, and Serotonergic Damage
Neurotoxicity Research (2017)
-
The effects of ecstasy on neurotransmitter systems: a review on the findings of molecular imaging studies
Psychopharmacology (2016)
-
Dopamine Function in Cigarette Smokers: An [18F]-DOPA PET Study
Neuropsychopharmacology (2014)
-
"Ecstasy"-induced toxicity in SH-SY5Y differentiated cells: role of hyperthermia and metabolites
Archives of Toxicology (2014)