Abstract
The mechanisms that coordinate and balance a complex network of opposing regulators to control Schwann cell (SC) differentiation remain elusive. Here we demonstrate that zinc-finger E-box-binding homeobox 2 (Zeb2, also called Sip1) transcription factor is a critical intrinsic timer that controls the onset of SC differentiation by recruiting histone deacetylases HDAC 1 and 2 (HDAC1/2) and nucleosome remodeling and deacetylase complex (NuRD) co-repressor complexes in mice. Zeb2 deletion arrests SCs at an undifferentiated state during peripheral nerve development and inhibits remyelination after injury. Zeb2 antagonizes inhibitory effectors including Notch and Sox2. Importantly, genome-wide transcriptome analysis reveals a Zeb2 target gene encoding the Notch effector Hey2 as a potent inhibitor for Schwann cell differentiation. Strikingly, a genetic Zeb2 variant associated with Mowat-Wilson syndrome disrupts the interaction with HDAC1/2–NuRD and abolishes Zeb2 activity for SC differentiation. Therefore, Zeb2 controls SC maturation by recruiting HDAC1/2–NuRD complexes and inhibiting a Notch–Hey2 signaling axis, pointing to the critical role of HDAC1/2–NuRD activity in peripheral neuropathies caused by ZEB2 mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
Change history
20 June 2016
In the version of this article initially published online, Danny Huylebroeck was listed under affiliation 2 (Institute of Pharmacology and Toxicology, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, China) instead of affiliation 3 (Department of Cell Biology, Erasmus University Medical Center, Rotterdam, the Netherlands). The error has been corrected for the print, PDF and HTML versions of this article.
References
Pereira, J.A., Lebrun-Julien, F. & Suter, U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 35, 123–134 (2012).
Nave, K.A. & Werner, H.B. Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol. 30, 503–533 (2014).
Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 7, a020529 (2015).
Dastot-Le Moal, F. et al. ZFHX1B mutations in patients with Mowat-Wilson syndrome. Hum. Mutat. 28, 313–321 (2007).
Mowat, D.R., Wilson, M.J. & Goossens, M. Mowat-Wilson syndrome. J. Med. Genet. 40, 305–310 (2003).
Nagaya, M., Kato, J., Niimi, N., Tanaka, S. & Wakamatsu, N. Clinical features of a form of Hirschsprung's disease caused by a novel genetic abnormality. J. Pediatr. Surg. 37, 1117–1122 (2002).
McKinsey, G.L. et al. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron 77, 83–98 (2013).
Weng, Q. et al. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron 73, 713–728 (2012).
van den Berghe, V. et al. Directed migration of cortical interneurons depends on the cell-autonomous action of Sip1. Neuron 77, 70–82 (2013).
Verstappen, G. et al. Atypical Mowat-Wilson patient confirms the importance of the novel association between ZFHX1B/SIP1 and NuRD corepressor complex. Hum. Mol. Genet. 17, 1175–1183 (2008).
Michailov, G.V. et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 (2004).
Taveggia, C. et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681–694 (2005).
Feltri, M.L. & Wrabetz, L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J. Peripher. Nerv. Syst. 10, 128–143 (2005).
Nodari, A. et al. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J. Neurosci. 28, 6714–6719 (2008).
Monk, K.R. et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325, 1402–1405 (2009).
Woodhoo, A. et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 12, 839–847 (2009).
Jaegle, M. et al. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 17, 1380–1391 (2003).
Topilko, P. et al. Krox-20 controls myelination in the peripheral nervous system. Nature 371, 796–799 (1994).
He, Y. et al. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat. Neurosci. 13, 1472–1480 (2010).
Svaren, J. & Meijer, D. The molecular machinery of myelin gene transcription in Schwann cells. Glia 56, 1541–1551 (2008).
Higashi, Y. et al. Generation of the floxed allele of the SIP1 (Smad-interacting protein 1) gene for Cre-mediated conditional knockout in the mouse. Genesis 32, 82–84 (2002).
Douglas, W.W. & Ritchie, J.M. Mammalian nonmyelinated nerve fibers. Physiol. Rev. 42, 297–334 (1962).
Doerflinger, N.H., Macklin, W.B. & Popko, B. Inducible site-specific recombination in myelinating cells. Genesis 35, 63–72 (2003).
Verschueren, K. et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J. Biol. Chem. 274, 20489–20498 (1999).
Jessen, K.R., Mirsky, R. & Lloyd, A.C. Schwann cells: development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 7, a020487 (2015).
Parrinello, S. et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143, 145–155 (2010).
Jessen, K.R. & Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6, 671–682 (2005).
Parkinson, D.B. et al. c-Jun is a negative regulator of myelination. J. Cell Biol. 181, 625–637 (2008).
Feltri, M.L., Poitelon, Y. & Previtali, S.C. How Schwann cells sort axons: new concepts. Neuroscientist 22, 252–265 (2016).
Fischer, A., Schumacher, N., Maier, M., Sendtner, M. & Gessler, M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 18, 901–911 (2004).
Xin, M. et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc. Natl. Acad. Sci. USA 104, 7975–7980 (2007).
Le, N. et al. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc. Natl. Acad. Sci. USA 102, 2596–2601 (2005).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Souilhol, C. et al. Nas transgenic mouse line allows visualization of Notch pathway activity in vivo. Genesis 44, 277–286 (2006).
De Strooper, B. et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999).
Pinnix, C.C. et al. Active Notch1 confers a transformed phenotype to primary human melanocytes. Cancer Res. 69, 5312–5320 (2009).
Lauberth, S.M. & Rauchman, M. A conserved 12-amino acid motif in Sall1 recruits the nucleosome remodeling and deacetylase corepressor complex. J. Biol. Chem. 281, 23922–23931 (2006).
Chen, Y. et al. HDAC-mediated deacetylation of NF-κB is critical for Schwann cell myelination. Nat. Neurosci. 14, 437–441 (2011).
Jacob, C. et al. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat. Neurosci. 14, 429–436 (2011).
van Grunsven, L.A. et al. XSip1 neuralizing activity involves the co-repressor CtBP and occurs through BMP dependent and independent mechanisms. Dev. Biol. 306, 34–49 (2007).
Quintes, S. et al. Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat. Neurosci. http://dx.doi.org/10.1038/nn.4321 (2016).
Conidi, A. et al. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signaling in vivo. Cytokine Growth Factor Rev. 22, 287–300 (2011).
Hung, H., Kohnken, R. & Svaren, J. The nucleosome remodeling and deacetylase chromatin remodeling (NuRD) complex is required for peripheral nerve myelination. J. Neurosci. 32, 1517–1527 (2012).
Desmazières, A., Decker, L., Vallat, J.M., Charnay, P. & Gilardi-Hebenstreit, P. Disruption of Krox20-Nab interaction in the mouse leads to peripheral neuropathy with biphasic evolution. J. Neurosci. 28, 5891–5900 (2008).
Reynolds, N., O'Shaughnessy, A. & Hendrich, B. Transcriptional repressors: multifaceted regulators of gene expression. Development 140, 505–512 (2013).
Fischer, A. et al. Hey basic helix-loop-helix transcription factors are repressors of GATA4 and GATA6 and restrict expression of the GATA target gene ANF in fetal hearts. Mol. Cell. Biol. 25, 8960–8970 (2005).
Doetzlhofer, A. et al. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell 16, 58–69 (2009).
Benito-Gonzalez, A. & Doetzlhofer, A. Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling. J. Neurosci. 34, 12865–12876 (2014).
Liu, Z., Owen, T., Fang, J., Srinivasan, R.S. & Zuo, J. In vivo Notch reactivation in differentiating cochlear hair cells induces Sox2 and Prox1 expression but does not disrupt hair cell maturation. Dev. Dyn. 241, 684–696 (2012).
Stanchina, L., Van de Putte, T., Goossens, M., Huylebroeck, D. & Bondurand, N. Genetic interaction between Sox10 and Zfhx1b during enteric nervous system development. Dev. Biol. 341, 416–428 (2010).
Brockes, J.P., Fields, K.L. & Raff, M.C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 165, 105–118 (1979).
Lu, Q.R. et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86 (2002).
Emig, D. et al. AltAnalyze and DomainGraph: analyzing and visualizing exon expression data. Nucleic Acids Res. 38, W755–62 (2010).
Salomonis, N. et al. Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proc. Natl. Acad. Sci. USA 107, 10514–10519 (2010).
Yu, Y. et al. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248–261 (2013).
Becker, W. Recent insights into the function of DYRK1A. FEBS J. 278, 222 (2011).
Trapnell, C., Pachter, L. & Salzberg, S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Ririe, D.G., Bremner, L.R. & Fitzgerald, M. Comparison of the immediate effects of surgical incision on dorsal horn neuronal receptive field size and responses during postnatal development. Anesthesiology 109, 698–706 (2008).
Marsh, D., Dickenson, A., Hatch, D. & Fitzgerald, M. Epidural opioid analgesia in infant rats I: mechanical and heat responses. Pain 82, 23–32 (1999).
Acknowledgements
The authors thank X. Chen and Z. Ma for technical support and initial observation of Zeb2 mutants. We thank A. Rauch for discussing ZEB2 missense mutations in MOWS patients, J. Svaren and E. Hurlock for suggestions and G. Verstappen and L. van Grunsven for initial study of Zeb2R22G–NuRD interactions. We are grateful to K.-A. Nave for communications of unpublished data. We also thank R. Kopan (University of Cincinnati) and A.J. Capiobianco (University of Miami) for the TP-1 reporter and lentiviral DN-MAML constructs and to J.W. Schneider and E.N. Olson (University of Texas Southwestern Medical Center)) for Notch-GFP reporter mice and Flag-HRT2/Hey2 vectors. This study was funded in part by grants from the US National Institute of Health R01NS072427 and R01NS075243 to Q.R.L., R01NS062796 to J.R.C. and R01AR064551 to M.P.J., and from the National Multiple Sclerosis Society (NMSS-4727) to Q.R.L. L.M.N.W. was supported by an NMSS Postdoctoral Fellowship (FA 2045A1/T). The work was also supported by Belspo grant IAP7-07 DevRepair, the Research Council of KU Leuven (GOA-11/012), FWO-V (G.0782.14), the type 3 large-infrastructure support InfraMouse by the Hercules Foundation (ZW09-03) and Erasmus MC start-up funds, to D.H.
Author information
Authors and Affiliations
Contributions
L.M.N.W. and Q.R.L. designed the experiments. L.M.N.W. carried out the studies. J.W., A.C. and L.Z. assisted with coimmunoprecipitation biochemical experiments for HDAC–NuRD and BMP–Smads. C. Zhao assisted with ChIP experiments. H.W. assisted with Notch-TP1 reporter assays. C. Zweier provided ZEB2 variant identification data. Z.F. assisted with heat hypersensitivity experiments. M.P.J. assisted with CMAP recordings. B.G.A., P.M., A.Z. and J.R.C. provided input and data interpretation. D.H. provided mice with the loxP-flanked Zeb2 allele and input on the study. L.M.N.W. and Q.R.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
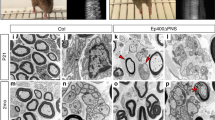
Supplementary Figure 1 Zeb2loxP/+;Dhh-cre peripheral nerves are comparable with wildtype or Zeb2loxP/loxP nerves
(a) Immunostaining showing Ki67 and Oct6 expression in P7 wildtype and Zeb2loxP/+;Dhh-cre longitudinal sciatic nerves. n = 3 animals/genotype. Scale bar: 50 µm. (b) Quantification of the proportions of Oct6+ (left) and Ki67+ (right) nuclei in P7 wildtype and Zeb2loxP/+;Dhh-cre sciatic nerves. Data are mean ± S.E.M.; P(Oct6) = 0.9896 t = 0.01390 df = 4; P(Ki67) = 0.7743 t = 0.3068 df = 4; Two-tailed unpaired Student’s t-test; n = 3 animals/genotype. (c) EM images showing unmyelinated axons in Remak bundles intercalated by SC processes in sciatic nerves of Zeb2loxP/loxP and Zeb2loxP/+;Dhh-cre mice in contrast to unsorted axon bundles in Zeb2loxP/loxP;Dhh-cre mice at 3 weeks. Scale bar: 2 µm. (d) A scatter plot showing g-ratio of myelinated axons of Zeb2loxP/loxP and Zeb2loxP/+;Dhh-cre sciatic nerves at 3 weeks. At least 100 axons per animal were counted. n = 3 animals/genotype. (e) g-ratios between Zeb2loxP/loxP and Zeb2loxP/+;Dhh-cre sciatic nerves at 3 weeks. n = 3 animals/genotype; data are mean ± S.E.M., P = 0.9272 t = 0.09727 df = 4; Two-tailed unpaired Student’s t-test. (f) Number of myelinated axons per area in sciatic nerves of Zeb2loxP/loxP and Zeb2loxP/+;Dhh-cre. Data are mean ± S.E.M., n = 3; Mann-Whitney test; P = 0.9000. (g) (Left) Number of unmyelinated axons per Remak bundle in sciatic nerves of 3-week-old Zeb2loxP/loxP and Zeb2loxP/+;Dhh-cre. Data are mean ± S.E.M., n = 3 animals/genotype; Mann-Whitney test; P = 0.4000. (Right) Percentage frequency distribution of the number of axons per Remak bundles in Zeb2loxP/loxP and Zeb2loxP/+;Dhh-cre sciatic nerves. (h) Zeb2loxP/loxP, Zeb2loxP/+;Dhh-cre and Zeb2loxP/loxP;Dhh-cre mice were tested for heat hypersensitivity to 50oC water 24h following carrageenan injection into the hairy hindpaw skin. Data are mean ± S.E.M., Zeb2loxP/loxP, n = 6; Zeb2loxP/+;Dhh-cre, n = 7; and Zeb2loxP/loxP;Dhh-cre, n = 4; *P < 0.05; **P < 0.01; Two-way repeated measures ANOVA with Sidak’s multiple comparisons test; Baseline: P > 0.05 Zeb2loxP/loxP vs Zeb2loxP/+;Dhh-cre; P > 0.05 Zeb2loxP/+;Dhh-cre vs Zeb2loxP/loxP;Dhh-cre, P > 0.05 Zeb2loxP/loxP vs Zeb2loxP/loxP;Dhh-cre; Carrageenan: P > 0.05 Zeb2loxP/loxP vs Zeb2loxP/+;Dhh-cre; P > 0.05 Zeb2loxP/+;Dhh-cre vs Zeb2loxP/loxP;Dhh-cre, P > 0.05 Zeb2loxP/loxP vs Zeb2loxP/loxP;Dhh-cre. Within animal group-baseline vs Carrageenan: Zeb2loxP/loxP P < 0.05; Zeb2loxP/+;Dhh-cre P < 0.01; Zeb2loxP/loxP;Dhh-cre P < 0.01. Interaction F (2, 14) = 0.7859; Time F (1, 14) = 44.03; Column Factor F (2, 14) = 0.1186; Subjects (matching) F (14, 14) = 2.49.
Supplementary Figure 2 Zeb2 is not required for myelin maintenance in SCs
(a) Survival curves of control (red) and Zeb2 cKO (black) mice (n = 40 animals/genotype). (b) Electron micrographs of cross sections of 5-month-old sciatic nerves from control (Zeb2loxP/+) and Zeb2 iKO (Zeb2loxP/loxP;Plp-creERT) induced by tamoxifen from 4 weeks old. n = 4. Scale bar: 2 µm.
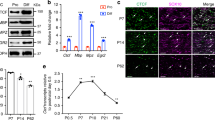
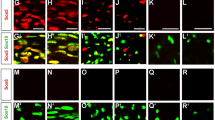
Supplementary Figure 3 siRNA-mediated Zeb2 knockdown causes a defect in SC differentiation
(a) qRT-PCR analysis showing expression of Zeb2, Oct6, Krox20, Mbp and Mpz in rat SCs transfected with scrambled and Zeb2 siRNA cultured in neuregulin-containing proliferation medium. (*P < 0.05, **P < 0.01; ***P < 0.001; n = 3 independent experiments; Data are presented as mean ± S.E.M., P(Zeb2) = 0.0001 t = 14.14 df = 4; P(Sox10) = 0.0416 t = 2.959 df = 4; P(Oct6) = 0.0003 t = 11.58 df = 4; P(Krox20) = 0.0072 t = 5.052 df = 4; P(Mbp) = 0.0077 t = 4.953 df = 4; P(Mpz) = 0.0037 t = 6.089 df = 4; Two-tailed unpaired Student’s t-test). (b) qRT-PCR analysis showing Oct6, Krox20, Mbp and Mpz expression in rat SCs transfected with scrambled and Zeb2 siRNA and induced to differentiate for 18 hours. (*P < 0.05, **P < 0.01; n = 3 independent experiments; Data are presented as mean ± S.E.M., P(Zeb2) = 0.0054 t = 5.489 df = 4; P(Oct6) = 0.0098 t = 5.891 df = 4; P(Krox20) = 0.0063 t = 5.254 df = 4; P(Mbp) = 0.0175 t = 3.905 df = 4; P(Mpz) = 0.0101 t = 4.586 df = 4; Two-tailed unpaired Student’s t-test). (c) ImmunoloxPuorescence labeling for differentiation markers, Oct6 and Krox20 in rat SCs transfected with scrambled and Zeb2 siRNA and induced to differentiate for 18 hours; n = 3 independent experiments. Scale bar: 50 µm. (d) Quantification of Oct6+ and Krox20+ cells relative to DAPI+ nuclei in scrambled and Zeb2-siRNA knockdown SCs induced to differentiate. Data are presented as mean ± S.E.M (***P < 0.001, n = 3 independent experiments; P(Oct6) < 0.0001 t = 40.4 df = 4; P(Krox20) = 0.0005 t = 10.31 df = 4; Two-tailed unpaired Student’s t-test). (e) ChIP assays using a Zeb2 antibody for its recruitment to the Zeb2 binding sites at -0.8kb and -3.9 kb from TSS in the Oct6 promoter were performed on chromatin from primary SCs exposed in proliferation or differentiation (9 h) media. IgG IP was used as control. Data are mean ± S.E.M; ***P < 0.001; n = 3 independent experiments; P(-0.8 kb) = 0.0061 t = 5.294 df = 4; P(-3.9 kb) = 0.0047 t = 4.367 df = 4; Two-tailed unpaired Student’s t-test.
Supplementary Figure 4 ChIP assays show that Zeb2 does not bind c-Jun promoter or to distal control regions of Sox2, Hey2 or Oct6 gene loci
(a) Zeb2 occupancy by ChIP–PCR in differentiating rat SCs on the promoter of c-Jun (-6 kb from TSS) shows no binding of Zeb2 to the putative Zeb2 binding site in the c-Jun promoter. n = 3 independent experiments; P = 0.9682 t = 0.04134 df = 7; Two-tailed unpaired Student’s t-test. (b) Distal control region of Sox2 is located at 25 kb upstream of TSS, that of Hey2 at 20 kb upstream of TSS and that of Oct6 at 18 kb. Input chromatin from rat SCs differentiated in the presence of cAMP for 9 hours shows amplification of the PCR fragment, Zeb2 binding in these regions was not detected in ChIP samples using anti-Zeb2 antibody. Full-length images are presented in Supplementary Figure 10d.
Supplementary Figure 5 Zeb2 regulates genes involved in the regulation of radial sorting
(a) Differential expression of selected genes involved in radial sorting regulation was detected based on transcriptome profiling of P7 control and Zeb2 cKO sciatic nerves. The table lists selected genes reported to inloxPuence radial sorting, their fold change in Zeb2 cKO sciatic nerves relative to control nerves and the sorting defects in corresponding mutants. Downregulated and upregulated genes are highlighted in red and blue, respectively. (b) qRT-PCR analysis of independently prepared P7 control and Zeb2 cKO sciatic nerves confirms the differential gene expression. Data are presented as mean ± S.E.M (**p < 0.01; ***p < 0.001; n = 3 independent experiments, P(Cdc42) = 0.0057 t = 5.402 df = 4; P(Dag1) = 0.0631 t = 2.553 df = 4; P(Itga6) = 0.001 t = 8.511 df = 4; P(Itga7) = 0.0004 t = 11.28 df = 4; P(Lama2) = 0.0007 t = 9.289 df = 4; Two-tailed unpaired Student’s t-test).
Supplementary Figure 6 Zeb2 deletion does not lead to activation of BMP signaling in sciatic nerves
Immunoblot analysis of p-Smad (1/5/8) and Smad7 in sciatic nerves of control and Zeb2 cKO mutants at P14. No increase in p-Smad (1/5/8) or alteration of Smad7 was detected in in Zeb2 cKO sciatic nerves. The p-Smad level appears to be even reduced in Zeb2 cKO sciatic nerves. GAPDH was used as a loading control. n = 3 independent experiments. Full-length blots are presented in Supplementary Figure 10c.
Supplementary Figure 7 Model schematics
(a) Strategy to detect Notch-mediated activation of eGFP expression in the transgenic Notch/RBP-J-RE-EGFP reporter line. (b) A schematic representation of the NuRD complex, composed of multiple subunits including the histone deacetylase core proteins HDAC1 and HDAC2, the histone-binding proteins RbAp46 and RbAp48, the chromatin remodeling ATPase Mi-2, the metastasis-associated proteins MTA1 (or MTA2/MTA3), the methyl-CpG-binding domain protein MBD3. (c) Schematic representation of SC lineage development and the dynamic expression of the transcription regulators as indicated. (d) A model depicts a dual mode of action by Zeb2 to promote SC differentiation by forming a transcriptional co-repressor complex with Hadc1/2-NuRD to inhibit differentiation inhibitory genes, e.g. Notch-Hey2 and Sox2 in pre-myelinating SCs, while activating pro-myelinating genes e.g. Oct6 and Krox20.
Supplementary Figure 8 Hey2 or Sox2 gene expression is not altered in Zeb2-deficient nerves after injury compared with controls
qRT-PCR analysis of Hey2 or Sox2 in transected distal sciatic nerves from adult control and Zeb2 iKO mice 7 days post-lesion showing no significant changes in mRNA levels. n = 3 animals/genotypes; data are presented as mean ± S.E.M; P(Zeb2) = 0.046 t = 2.422 df = 7; P(Hey2) = 0.3864 t = 0.9713 df = 4; P(Sox2) = 0.4095 t = 0.9203 df = 4; Two-tailed unpaired Student’s t-test.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1949 kb)
Rights and permissions
About this article
Cite this article
Wu, L., Wang, J., Conidi, A. et al. Zeb2 recruits HDAC–NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat Neurosci 19, 1060–1072 (2016). https://doi.org/10.1038/nn.4322
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4322
This article is cited by
-
Histone H3.3 K27M chromatin functions implicate a network of neurodevelopmental factors including ASCL1 and NEUROD1 in DIPG
Epigenetics & Chromatin (2022)
-
Sodium phenylbutyrate inhibits Schwann cell inflammation via HDAC and NFκB to promote axonal regeneration and remyelination
Journal of Neuroinflammation (2021)
-
Schwann cell plasticity regulates neuroblastic tumor cell differentiation via epidermal growth factor-like protein 8
Nature Communications (2021)
-
Peripheral Nerve Development and the Pathogenesis of Peripheral Neuropathy: the Sorting Point
Neurotherapeutics (2021)
-
Emerging molecular subtypes and therapeutic targets in B-cell precursor acute lymphoblastic leukemia
Frontiers of Medicine (2021)