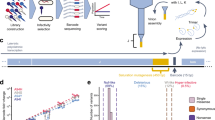
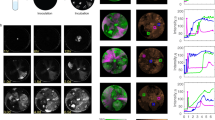
Abstract
The emergence of mutations following growth-limiting conditions underlies bacterial drug resistance, viral escape from the immune system and fundamental evolution-driven events. Intriguingly, whether mutations are induced by growth limitation conditions or are randomly generated during growth and then selected by growth limitation conditions remains an open question1. Here, we show that bacteriophage T7 undergoes apparent stress-induced mutagenesis when selected for improved recognition of its host's receptor. In our unique experimental set-up, the growth limitation condition is physically and temporally separated from mutagenesis: growth limitation occurs while phage DNA is outside the host, and spontaneous mutations occur during phage DNA replication inside the host. We show that the selected beneficial mutations are not pre-existing and that the initial slow phage growth is enabled by the phage particle's low-efficiency DNA injection into the host. Thus, the phage particle allows phage populations to initially extend their host range without mutagenesis by virtue of residual recognition of the host receptor. Mutations appear during non-selective intracellular replication, and the frequency of mutant phages increases by natural selection acting on free phages, which are not capable of mutagenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Maisnier-Patin, S. & Roth, J. R. The origin of mutants under selection: how natural selection mimics mutagenesis (adaptive mutation). Cold Spring Harb. Perspect Biol. 7, a018176 (2015).
Lederberg, J. & Lederberg, E. M. Replica plating and indirect selection of bacterial mutants. J. Bacteriol. 63, 399–406 (1952).
Newcombe, H. B. Origin of bacterial variants. Nature 164, 150–151 (1949).
Luria, S. E. & Delbruck, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Cairns, J. & Foster, P. L. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128, 695–701 (1991).
Cairns, J., Overbaugh, J. & Miller, S. The origin of mutants. Nature 335, 142–145 (1988).
Bjedov, I. et al. Stress-induced mutagenesis in bacteria. Science 300, 1404–1409 (2003).
Slack, A., Thornton, P. C., Magner, D. B., Rosenberg, S. M. & Hastings, P. J. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2, e48 (2006).
Wrande, M., Roth, J. R. & Hughes, D. Accumulation of mutants in ‘aging’ bacterial colonies is due to growth under selection, not stress-induced mutagenesis. Proc. Natl Acad. Sci. USA 105, 11863–11868 (2008).
Roth, J. R. The joys and terrors of fast adaptation: new findings elucidate antibiotic resistance and natural selection. Mol. Microbiol. 79, 279–282 (2011).
Quinones-Soto, S., Reams, A. B. & Roth, J. R. Pathways of genetic adaptation: multi-step origin of mutants under selection without induced mutagenesis in Salmonella enterica. Genetics 192, 987–999 (2012).
Sano, E., Maisnier-Patin, S., Aboubechara, J. P., Quinones-Soto, S. & Roth, J. R. Plasmid copy number underlies adaptive mutability in bacteria. Genetics 198, 919–933 (2014).
Reams, A. B., Kofoid, E., Savageau, M. & Roth, J. R. Duplication frequency in a population of Salmonella enterica rapidly approaches steady state with or without recombination. Genetics 184, 1077–1094 (2010).
Petrosino, J. F., Galhardo, R. S., Morales, L. D. & Rosenberg, S. M. Stress-induced β-lactam antibiotic resistance mutation and sequences of stationary-phase mutations in the Escherichia coli chromosome. J. Bacteriol. 191, 5881–5889 (2009).
Roth, J. R. Genetic adaptation: a new piece for a very old puzzle. Curr. Biol. 20, R15–R17 (2010).
Qimron, U., Marintcheva, B., Tabor, S. & Richardson, C. C. Genomewide screens for Escherichia coli genes affecting growth of T7 bacteriophage. Proc. Natl Acad. Sci. USA 103, 19039–19044 (2006).
Molineux, I. J. in The Bacteriophages (eds Abedon, S. T. & Calendar, R. L. ) 275–299 (Oxford Univ. Press, 2005).
Drake, J. W. A constant rate of spontaneous mutation in DNA-based microbes. Proc. Natl Acad. Sci. USA 88, 7160–7164 (1991).
Rosenberg, A. H., Patel, S. S., Johnson, K. A. & Studier, F. W. Cloning and expression of gene 4 of bacteriophage T7 and creation and analysis of T7 mutants lacking the 4A primase/helicase or the 4B helicase. J. Biol. Chem. 267, 15005–15012 (1992).
Lee, S. J. & Richardson, C. C. Essential lysine residues in the RNA polymerase domain of the gene 4 primase-helicase of bacteriophage T7. J. Biol. Chem. 276, 49419–49426 (2001).
Chung, Y. B. & Hinkle, D. C. Bacteriophage T7 DNA packaging. I. Plasmids containing a T7 replication origin and the T7 concatemer junction are packaged into transducing particles during phage infection. J. Mol. Biol. 216, 911–926 (1990).
Struthers-Schlinke, J. S., Robins, W. P., Kemp, P. & Molineux, I. J. The internal head protein Gp16 controls DNA ejection from the bacteriophage T7 virion. J. Mol. Biol. 301, 35–45 (2000).
Molineux, I. J. No syringes please, ejection of phage T7 DNA from the virion is enzyme driven. Mol. Microbiol. 40, 1–8 (2001).
Garcia, L. R. & Molineux, I. J. Transcription-independent DNA translocation of bacteriophage T7 DNA into Escherichia coli. J. Bacteriol. 178, 6921–6929 (1996).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Acknowledgements
The authors thank C. Vainstein for professional language editing and I.J. Molineux for providing D352-836 T7 phage. The study was supported by the European Research Council StG programme (grant no. 336079 to U.Q.), the Israel Science Foundation (grant no. 268/14 to U.Q., grant no. 1303/12 to R.S. and I-CORE grant no. 1796 to R.S.) and the Israeli Ministry of Health (grant no. 9988-3 to U.Q.). A.L. acknowledges the Azrieli Foundation for the award of an Azrieli Fellowship.
Author information
Authors and Affiliations
Contributions
I.Y., R.E. and U.Q. conceived and designed the experiments. I.Y., R.E. and G.A. performed the experiments. I.Y., R.E., A.L., R.S., A.M. and U.Q. analysed the data. U.Q. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Figures (PDF 357 kb)
Supplementary Table 3
Mutation frequencies in each nucleotide of bacteriophage T7 genomes grown on the indicated hosts. (XLSX 2738 kb)
Rights and permissions
About this article
Cite this article
Yosef, I., Edgar, R., Levy, A. et al. Natural selection underlies apparent stress-induced mutagenesis in a bacteriophage infection model. Nat Microbiol 1, 16047 (2016). https://doi.org/10.1038/nmicrobiol.2016.47
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.47