Abstract
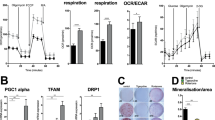
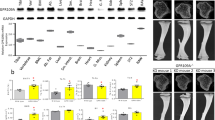
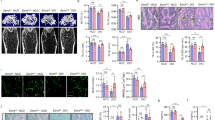
Peroxisome proliferator-activated receptors (PPARs) act as metabolic sensors and central regulators of fat and glucose homeostasis1. Furthermore, PPARγ has been implicated as major catabolic regulator of bone mass in mice and humans2,3,4,5. However, a potential involvement of other PPAR subtypes in the regulation of bone homeostasis has remained elusive. Here we report a previously unrecognized role of PPARβ/δ as a key regulator of bone turnover and the crosstalk between osteoblasts and osteoclasts. In contrast to activation of PPARγ, activation of PPARβ/δ amplified Wnt-dependent and β-catenin–dependent signaling and gene expression in osteoblasts, resulting in increased expression of osteoprotegerin (OPG) and attenuation of osteoblast-mediated osteoclastogenesis. Accordingly, PPARβ/δ-deficient mice had lower Wnt signaling activity, lower serum concentrations of OPG, higher numbers of osteoclasts and osteopenia. Pharmacological activation of PPARβ/δ in a mouse model of postmenopausal osteoporosis led to normalization of the altered ratio of tumor necrosis factor superfamily, member 11 (RANKL, also called TNFSF11) to OPG, a rebalancing of bone turnover and the restoration of normal bone density. Our findings identify PPARβ/δ as a promising target for an alternative approach in the treatment of osteoporosis and related diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Evans, R.M., Barish, G.D. & Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 10, 355–361 (2004).
Kawai, M. & Rosen, C.J. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 6, 629–636 (2010).
Giaginis, C., Tsantili-Kakoulidou, A. & Theocharis, S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam. Clin. Pharmacol. 21, 231–244 (2007).
Viccica, G., Francucci, C.M. & Marcocci, C. The role of PPARγ for the osteoblastic differentiation. J. Endocrinol. Invest. 33, 9–12 (2010).
Grey, A. Skeletal consequences of thiazolidinedione therapy. Osteoporos. Int. 19, 129–137 (2008).
Akune, T. et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113, 846–855 (2004).
Wan, Y., Chong, L.W. & Evans, R.M. PPAR-γ regulates osteoclastogenesis in mice. Nat. Med. 13, 1496–1503 (2007).
Wei, W. et al. PGC1β mediates PPARγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 11, 503–516 (2010).
Takada, I., Kouzmenko, A.P. & Kato, S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 5, 442–447 (2009).
Mulholland, D.J., Dedhar, S., Coetzee, G.A. & Nelson, C.C. Interaction of nuclear receptors with the Wnt/β-catenin/Tcf signaling axis: Wnt you like to know? Endocr. Rev. 26, 898–915 (2005).
Takada, I., Suzawa, M., Matsumoto, K. & Kato, S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann. NY Acad. Sci. 1116, 182–195 (2007).
Okamura, M. et al. COUP-TFII acts downstream of Wnt/β-catenin signal to silence PPARγ gene expression and repress adipogenesis. Proc. Natl. Acad. Sci. USA 106, 5819–5824 (2009).
Kubota, T., Michigami, T. & Ozono, K. Wnt signaling in bone metabolism. J. Bone Miner. Metab. 27, 265–271 (2009).
Glass, D.A. II et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 (2005).
Holmen, S.L. et al. Essential role of β-catenin in postnatal bone acquisition. J. Biol. Chem. 280, 21162–21168 (2005).
Kramer, I. et al. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 30, 3071–3085 (2010).
Wang, D., Mann, J.R. & DuBois, R.N. WNT and cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle 3, 1512–1515 (2004).
Wang, D. et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ. Cancer Cell 6, 285–295 (2004).
Nadra, K. et al. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor β/δ. Mol. Cell Biol. 26, 3266–3281 (2006).
Hayashi, S., Lewis, P., Pevny, L. & McMahon, A.P. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech. Dev. 119, S97–S101 (2002).
Cui, Y. et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 17, 684–691 (2011).
Cho, E.S. et al. The effects of rosiglitazone on osteoblastic differentiation, osteoclast formation and bone resorption. Mol. Cells 33, 173–181 (2012).
Chan, B.Y. et al. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone 40, 149–159 (2007).
Barish, G.D., Narkar, V.A. & Evans, R.M. PPARδ: a dagger in the heart of the metabolic syndrome. J. Clin. Invest. 116, 590–597 (2006).
Narkar, V.A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Lee, N.K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007).
Still, K., Grabowski, P., Mackie, I., Perry, M. & Bishop, N. The peroxisome proliferator activator receptor α/δ agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif. Tissue Int. 83, 285–292 (2008).
Olson, E.J., Pearce, G.L., Jones, N.P. & Sprecher, D.L. Lipid effects of peroxisome proliferator-activated receptor-δ agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 32, 2289–2294 (2012).
Hayashi, S., Lewis, P., Pevny, L. & McMahon, A.P. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene expression patterns. Gene Expr. Patterns 2, 93–97 (2002).
Kleyer, A. et al. LXRs orchestrate osteoblast/osteoclast crosstalk and counteract pathologic bone loss. J. Bone Miner. Res. 27, 2442–2451 (2012).
Krönke, G. et al. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 62, 2303–2312 (2010).
Bouffi, C., Bony, C., Courties, G., Jorgensen, C. & Noël, D. IL-6–dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE 5, e14247 (2010).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Krönke, G. et al. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J. Biol. Chem. 278, 51006–51014 (2003).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Acknowledgements
We thank M. Steffen, U. Hillienhoff and I. Schmidt for technical assistance and J. Wittmann for his help during the analysis of the Lrp5 promoter. Wnt3a-expressing L cells were kindly provided by K. Tanneberger (Department of Experimental Medicine II, University of Erlangen). This study was supported by the Deutsche Forschungsgemeinschaft (KR3523, FG 661, SFB643 and SPP1468-IMMUNOBONE), the Bundesministerium für Bildung und Forschung (BMBF; project ANCYLOSS); the ELAN Program of the University of Erlangen-Nuremberg; the MASTERSWITCH and IMI projects of the European Union; the Interdisciplinary Centre for Clinical Research (IZKF) Erlangen; and the Bayrische Forschungsstftung.
Author information
Authors and Affiliations
Contributions
C. Scholtysek planned and performed in vitro and in vivo experiments, conducted data analyses and wrote the manuscript. J.K., H.F., S.U. and M.M.Z. conducted data analyses and performed in vivo and in vitro experiments. N.I. and F.D. performed MSC differentiation and characterization and conducted the related experiments and data analyses. C. Stoll cloned and characterized the Lrp5 promoter. M.S., L.D., C.B. and A.K. performed histological data acquisition and data analyses. A.H., K.E. and J.P.T. established, performed and interpreted the microcomputer tomography–based bone analyses. B.D. generated and provided PpardSox2-cKO mice and contributed to the study design. J.-P.D. and G.S. provided input and wrote the manuscript. G.K. supervised the project, planned and conducted experiments and data analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
(PDF 867 kb)
Rights and permissions
About this article
Cite this article
Scholtysek, C., Katzenbeisser, J., Fu, H. et al. PPARβ/δ governs Wnt signaling and bone turnover. Nat Med 19, 608–613 (2013). https://doi.org/10.1038/nm.3146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3146
This article is cited by
-
An AMP Kinase-pathway dependent integrated stress response regulates ageing and longevity
Biogerontology (2023)
-
PPARβ/δ priming enhances the anti-apoptotic and therapeutic properties of mesenchymal stromal cells in myocardial ischemia–reperfusion injury
Stem Cell Research & Therapy (2022)
-
Acetyl-CoA-Carboxylase 1-mediated de novo fatty acid synthesis sustains Lgr5+ intestinal stem cell function
Nature Communications (2022)
-
The roles of osteoprotegerin in cancer, far beyond a bone player
Cell Death Discovery (2022)
-
Emerging Therapeutic Potential of Short Mitochondrial-produced Peptides for Anabolic Osteogenesis
International Journal of Peptide Research and Therapeutics (2022)