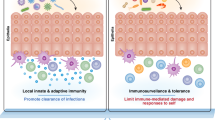
Abstract
Allergic inflammation develops in tissues that have large epithelial surface areas that are exposed to the environment, such as the lung, skin and gut. In the steady state, antigen-experienced memory T cells patrol these peripheral tissues to facilitate swift immune responses against invading pathogens. In at least two allergy-prone organs, the skin and the gut, memory T cells are programmed during the initial antigen priming to express trafficking receptors that enable them to preferentially home to these organs. In this review we propose that tissue-specific memory and inflammation-specific T cell trafficking facilitates the development of allergic disease in these organs. We thus review recent advances in our understanding of tissue-specific T cell trafficking and how regulation of T cell trafficking by the chemokine system contributes to allergic inflammation in mouse models and in human allergic diseases of the skin, lung and gut. Inflammation- and tissue-specific T lymphocyte trafficking pathways are currently being targeted as new treatments for non-allergic inflammatory diseases and may yield effective new therapeutics for allergic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cookson, W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat. Rev. Immunol. 4, 978–988 (2004).
Rodriguez, E. et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J. Allergy Clin. Immunol. 123, 1361–1370 (2009).
Al-Shami, A. et al. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202, 829–839 (2005).
Eiwegger, T. & Akdis, C.A. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur. J. Immunol. 41, 1535–1538 (2011).
Fort, M.M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Hvid, M. et al. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J. Invest. Dermatol. 131, 150–157 (2011).
Paul, W.E. & Zhu, J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 10, 225–235 (2010).
Préfontaine, D. et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 183, 5094–5103 (2009).
Rank, M.A. et al. IL-33–activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 123, 1047–1054 (2009).
Luster, A.D., Alon, R. & von Andrian, U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182–1190 (2005).
Agace, W.W. Tissue-tropic effector T cells: generation and targeting opportunities. Nat. Rev. Immunol. 6, 682–692 (2006).
Bromley, S.K., Mempel, T.R. & Luster, A.D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9, 970–980 (2008).
Sallusto, F. & Mackay, C.R. Chemoattractants and their receptors in homeostasis and inflammation. Curr. Opin. Immunol. 16, 724–731 (2004).
Sallusto, F., Mackay, C.R. & Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18, 593–620 (2000).
Sallusto, F. et al. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187, 875–883 (1998).
Cahill, R.N. et al. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J. Exp. Med. 145, 420–428 (1977).
Berg, E.L. et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 174, 1461–1466 (1991).
Berlin, C. et al. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80, 413–422 (1995).
Picker, L.J. et al. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature 349, 796–799 (1991).
Picker, L.J. et al. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J. Immunol. 145, 3247–3255 (1990).
Sigmundsdottir, H. & Butcher, E.C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 9, 981–987 (2008).
Briskin, M. et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 151, 97–110 (1997).
Kunkel, E.J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Campbell, J.J. et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400, 776–780 (1999).
Duhen, T. et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10, 857–863 (2009).
Fuhlbrigge, R.C. et al. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 389, 978–981 (1997).
Homey, B. et al. CCL27-CCR10 interactions regulate T cell–mediated skin inflammation. Nat. Med. 8, 157–165 (2002).
Islam, S.A. et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ TH2 cells. Nat. Immunol. 12, 167–177 (2011).
Schaerli, P. et al. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 199, 1265–1275 (2004).
Trifari, S. et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 10, 864–871 (2009).
Clark, R.A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Charbonnier, A.S. et al. Macrophage inflammatory protein 3α is involved in the constitutive trafficking of epidermal langerhans cells. J. Exp. Med. 190, 1755–1768 (1999).
Gombert, M. et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J. Immunol. 174, 5082–5091 (2005).
Morales, J. et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl. Acad. Sci. USA 96, 14470–14475 (1999).
Weninger, W. et al. Specialized contributions by α(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity 12, 665–676 (2000).
Annacker, O. et al. Essential role for CD103 in the T cell–mediated regulation of experimental colitis. J. Exp. Med. 202, 1051–1061 (2005).
Edele, F. et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J. Immunol. 181, 3745–3749 (2008).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Mora, J.R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Mora, J.R. et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201, 303–316 (2005).
Sigmundsdottir, H. et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 8, 285–293 (2007).
Ohmichi, Y. et al. Essential role of peripheral node addressin in lymphocyte homing to nasal-associated lymphoid tissues and allergic immune responses. J. Exp. Med. 208, 1015–1025 (2011).
de Bree, G.J. et al. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 202, 1433–1442 (2005).
Koelle, D.M. et al. Expression of cutaneous lymphocyte–associated antigen by CD8+ T cells specific for a skin-tropic virus. J. Clin. Invest. 110, 537–548 (2002).
Campbell, J.J. et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J. Immunol. 166, 2842–2848 (2001).
Purwar, R. et al. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE 6, e16245 (2011).
Thomas, S.Y. et al. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J. Immunol. 179, 1901–1912 (2007).
Sheridan, B.S. & Lefrancois, L. Regional and mucosal memory T cells. Nat. Immunol. 121, 485–491 (2011).
Sallusto, F. et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Reinhardt, R.L. et al. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Conrad, C. et al. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 13, 836–842 (2007).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Klonowski, K.D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Vezys, V. et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457, 196–199 (2009).
Wakim, L.M. et al. Dendritic cell–induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Lambrecht, B.N. & Hammad, H. Biology of lung dendritic cells at the origin of asthma. Immunity 31, 412–424 (2009).
Adorini, L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann. NY Acad. Sci. 987, 258–261 (2003).
Benson, M.J. et al. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 204, 1765–1774 (2007).
Boonstra, A. et al. 1α,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 167, 4974–4980 (2001).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid–dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Iwata, M., Eshima, Y. & Kagechika, H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 15, 1017–1025 (2003).
Sun, C.M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011).
DePaolo, R.W. et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471, 220–224 (2011).
Iellem, A. et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194, 847–853 (2001).
Siegmund, K. et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 106, 3097–3104 (2005).
Worbs, T. et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527 (2006).
Worbs, T. & Forster, R. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol. 28, 274–280 (2007).
Zammit, D.J. et al. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 24, 439–449 (2006).
McLachlan, J.B. et al. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 30, 277–288 (2009).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Harris, N.L. et al. Differential T cell function and fate in lymph node and nonlymphoid tissues. J. Exp. Med. 195, 317–326 (2002).
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Ansel, K.M., Lee, D.U. & Rao, A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 4, 616–623 (2003).
Sallusto, F., Lanzavecchia, A. & Mackay, C.R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today 19, 568–574 (1998).
Abe, H. et al. Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene 227, 71–77 (1999).
Hirai, H. et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193, 255–261 (2001).
Upadhyaya, B. et al. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J. Immunol. 187, 3111–3120 (2011).
Kim, C.H., Nagata, K. & Butcher, E.C. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J. Immunol. 171, 152–158 (2003).
D'Ambrosio, D. et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J. Immunol. 161, 5111–5115 (1998).
Wei, L. et al. Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation. Immunity 32, 840–851 (2010).
Meisel, C. et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J. Immunol. 166, 3143–3150 (2001).
Monney, L. et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002).
Hegazy, A.N. et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 32, 116–128 (2010).
Wang, Y.H. et al. A novel subset of CD4+ T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 207, 2479–2491 (2010).
Zhu, J. & Paul, W.E. CD4+ T cell plasticity-Th2 cells join the crowd. Immunity 32, 11–13 (2010).
Corren, J. et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365, 1088–1098 (2011).
Kraft, M. Asthma phenotypes and interleukin-13—moving closer to personalized medicine. N. Engl. J. Med. 365, 1141–1144 (2011).
Kim, C.H. et al. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108, 1331–1339 (2001).
Leung, D.Y. & Bieber, T. Atopic dermatitis. Lancet 361, 151–160 (2003).
Woodward, A.L. et al. An obligate role for T-cell receptor αβ+ T cells but not T-cell receptor γδ+ T cells, B cells, or CD40/CD40L interactions in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 107, 359–366 (2001).
Kakinuma, T. et al. Increased serum cutaneous T cell–attracting chemokine (CCL27) levels in patients with atopic dermatitis and psoriasis vulgaris. J. Allergy Clin. Immunol. 111, 592–597 (2003).
Leung, T.F. et al. Plasma concentration of thymus and activation-regulated chemokine is elevated in childhood asthma. J. Allergy Clin. Immunol. 110, 404–409 (2002).
Shimada, Y., Takehara, K. & Sato, S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 34, 201–208 (2004).
Vestergaard, C. et al. Expression of the T-helper 2–specific chemokine receptor CCR4 on CCR10-positive lymphocytes in atopic dermatitis skin but not in psoriasis skin. Br. J. Dermatol. 149, 457–463 (2003).
Lonsdorf, A.S., Hwang, S.T. & Enk, A.H. Chemokine receptors in T-cell–mediated diseases of the skin. J. Invest. Dermatol. 129, 2552–2566 (2009).
Pivarcsi, A. et al. CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J. Immunol. 173, 5810–5817 (2004).
Günther, C. et al. CCL18 is expressed in atopic dermatitis and mediates skin homing of human memory T cells. J. Immunol. 174, 1723–1728 (2005).
Hijnen, D. et al. Differential expression of genes involved in skin homing, proliferation, and apoptosis in CD4+ T cells of patients with atopic dermatitis. J. Invest. Dermatol. 125, 1149–1155 (2005).
Hijnen, D. et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell- attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J. Allergy Clin. Immunol. 113, 334–340 (2004).
Santamaria Babi, L.F. et al. Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J. Exp. Med. 181, 1935–1940 (1995).
Echigo, T. et al. Expression of fractalkine and its receptor, CX3CR1, in atopic dermatitis: possible contribution to skin inflammation. J. Allergy Clin. Immunol. 113, 940–948 (2004).
Mionnet, C. et al. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat. Med. 16, 1305–1312 (2010).
Tremblay, K. et al. Association study between the CX3CR1 gene and asthma. Genes Immun. 7, 632–639 (2006).
Spergel, J.M. et al. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Invest. 103, 1103–1111 (1999).
Oyoshi, M.K. et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J. Clin. Invest. 121, 2210–2220 (2011).
Campbell, J.J., O'Connell, D.J. & Wurbel, M.A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 178, 3358–3362 (2007).
Takatsu, K. & Nakajima, H. IL-5 and eosinophilia. Curr. Opin. Immunol. 20, 288–294 (2008).
Lloyd, C.M. & Hessel, E.M. Functions of T cells in asthma: more than just TH2 cells. Nat. Rev. Immunol. 10, 838–848 (2010).
Robinson, D.S. The role of the T cell in asthma. J. Allergy Clin. Immunol. 126, 1081–1091, quiz 1092–1093 (2010).
Fairs, A. et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am. J. Respir. Crit. Care Med. 182, 1362–1368 (2010).
Kheradmand, F. et al. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169, 5904–5911 (2002).
Lamhamedi-Cherradi, S.E. et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J. Immunol. 180, 6000–6009 (2008).
Wark, P.A. et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201, 937–947 (2005).
Muro, S., Minshall, E.M. & Hamid, Q.A. The pathology of chronic asthma. Clin. Chest Med. 21, 225–244 (2000).
Bentley, A.M., Kay, A.B. & Durham, S.R. Human late asthmatic reactions. Clin. Exp. Allergy 27 (suppl. 1), 71–86 (1997).
Mathew, A. et al. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J. Exp. Med. 193, 1087–1096 (2001).
Mathew, A. et al. Cutting edge: Th2 cell trafficking into the allergic lung is dependent on chemoattractant receptor signaling. J. Immunol. 169, 651–655 (2002).
Medoff, B.D., Thomas, S.Y. & Luster, A.D. T cell trafficking in allergic asthma: the ins and outs. Annu. Rev. Immunol. 26, 205–232 (2008).
Tager, A.M. et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat. Immunol. 4, 982–990 (2003).
Medoff, B.D. et al. Antibody-antigen interaction in the airway drives early granulocyte recruitment through BLT1. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L170–L178 (2006).
Gonzalo, J.A. et al. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J. Immunol. 179, 1740–1750 (2007).
Oliveira, S.H. & Lukacs, N.W. Stem cell factor and igE-stimulated murine mast cells produce chemokines (CCL2, CCL17, CCL22) and express chemokine receptors. Inflamm. Res. 50, 168–174 (2001).
Cameron, L. et al. Genetic variation in CRTh2 influences development of allergic phenotypes. Allergy 64, 1478–1485 (2009).
Huang, J.L. et al. Sequence variants of the gene encoding chemoattractant receptor expressed on Th2 cells (CRTH2) are associated with asthma and differentially influence mRNA stability. Hum. Mol. Genet. 13, 2691–2697 (2004).
Pichavant, M. et al. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J. Allergy Clin. Immunol. 115, 771–778 (2005).
Kawai, T. et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167, 5887–5894 (2001).
Reibman, J. et al. Airway epithelial cells release MIP-3α/CCL20 in response to cytokines and ambient particulate matter. Am. J. Respir. Cell Mol. Biol. 28, 648–654 (2003).
Lukacs, N.W. et al. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J. Exp. Med. 194, 551–555 (2001).
Lundy, S.K. et al. Attenuation of allergen-induced responses in CCR6−/− mice is dependent upon altered pulmonary T lymphocyte activation. J. Immunol. 174, 2054–2060 (2005).
Weckmann, M. et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat. Med. 13, 1308–1315 (2007).
Medoff, B.D. et al. IFN-γ–inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J. Immunol. 168, 5278–5286 (2002).
Panina-Bordignon, P. et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 107, 1357–1364 (2001).
Nouri-Aria, K.T. et al. CCR4 in human allergen-induced late responses in the skin and lung. Eur. J. Immunol. 32, 1933–1938 (2002).
Pilette, C. et al. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur. Respir. J. 23, 876–884 (2004).
Mutalithas, K. et al. Expression of CCR8 is increased in asthma. Clin. Exp. Allergy 40, 1175–1185 (2010).
Chensue, S.W. et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J. Exp. Med. 193, 573–584 (2001).
Schuh, J.M. et al. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J. 16, 1313–1315 (2002).
Chung, C.D. et al. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J. Immunol. 170, 581–587 (2003).
Conroy, D.M. et al. CCR4 blockade does not inhibit allergic airways inflammation. J. Leukoc. Biol. 74, 558–563 (2003).
Goya, I. et al. Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J. Immunol. 170, 2138–2146 (2003).
Mikhak, Z. et al. Contribution of CCR4 and CCR8 to antigen-specific TH2 cell trafficking in allergic pulmonary inflammation. J. Allergy Clin. Immunol. 123, 67–73 (2009).
Vijayanand, P. et al. Chemokine receptor 4 plays a key role in T cell recruitment into the airways of asthmatic patients. J. Immunol. 184, 4568–4574 (2010).
El-Shazly, A. et al. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J. Immunol. 176, 1860–1868 (2006).
Van Snick, J. et al. I-309/T cell activation gene-3 chemokine protects murine T cell lymphomas against dexamethasone-induced apoptosis. J. Immunol. 157, 2570–2576 (1996).
Medoff, B.D. et al. CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J. Immunol. 182, 623–635 (2009).
Perros, F. et al. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy 64, 995–1002 (2009).
Voehringer, D. et al. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203, 1435–1446 (2006).
Fulkerson, P.C. et al. Pulmonary chemokine expression is coordinately regulated by STAT1, STAT6, and IFN-γ. J. Immunol. 173, 7565–7574 (2004).
Reekers, R. et al. The role of circulating food antigen-specific lymphocytes in food allergic children with atopic dermatitis. Br. J. Dermatol. 135, 935–941 (1996).
Beyer, K. et al. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J. Allergy Clin. Immunol. 109, 707–713 (2002).
Lin, X.P. et al. Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J. Allergy Clin. Immunol. 109, 879–887 (2002).
Eigenmann, P.A., Tropia, L. & Hauser, C. The mucosal adhesion receptor α4β7 integrin is selectively increased in lymphocytes stimulated with β-lactoglobulin in children allergic to cow's milk. J. Allergy Clin. Immunol. 103, 931–936 (1999).
Schulten, V. et al. Characterization of the allergic T-cell response to Pru p 3, the nonspecific lipid transfer protein in peach. J. Allergy Clin. Immunol. 124, 100–107 (2009).
Abernathy-Carver, K.J. et al. Milk-induced eczema is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J. Clin. Invest. 95, 913–918 (1995).
Beyer, K. et al. Milk-induced urticaria is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J. Allergy Clin. Immunol. 109, 688–693 (2002).
Prussin, C., Lee, J. & Foster, B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5− TH2 responses. J. Allergy Clin. Immunol. 124, 1326–1332 (2009).
Delong, J.H. et al. Ara h 1-reactive T cells in individuals with peanut allergy. J. Allergy Clin. Immunol. 127, 1211–1218 (2011).
Knight, A.K. et al. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G1234–G1243 (2007).
Blazquez, A.B. et al. A functional role for CCR6 on proallergic T cells in the gastrointestinal tract. Gastroenterology 138, 275–284 (2010).
Harper, E.G. et al. Efalizumab therapy for atopic dermatitis causes marked increases in circulating effector memory CD4+ T cells that express cutaneous lymphocyte antigen. J. Invest. Dermatol. 128, 1173–1181 (2008).
Pettipher, R., Hansel, T.T. & Armer, R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat. Rev. Drug Discov. 6, 313–325 (2007).
Carson, K.R. et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 10, 816–824 (2009).
Acknowledgements
The authors were supported by US National Institutes of Health grants R37AI040618 and U19AI095261 to A.D.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Islam, S., Luster, A. T cell homing to epithelial barriers in allergic disease. Nat Med 18, 705–715 (2012). https://doi.org/10.1038/nm.2760
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2760
This article is cited by
-
Inhibition of miR-99a-5p prevents allergen-driven airway exacerbations without compromising type-2 memory responses in the intestine following helminth infection
Mucosal Immunology (2021)
-
What does elevated TARC/CCL17 expression tell us about eosinophilic disorders?
Seminars in Immunopathology (2021)
-
Lack of interleukin-33 and its receptor does not prevent calcipotriol-induced atopic dermatitis-like inflammation in mice
Scientific Reports (2020)
-
Sialyl Glycan Expression on T Cell Subsets in Asthma: a correlation with disease severity and blood parameters
Scientific Reports (2019)
-
Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases
Nature Communications (2019)