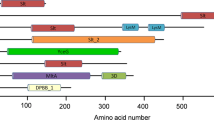
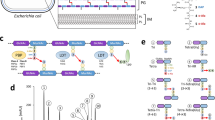
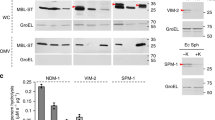
Abstract
The peptidoglycan layer is a vital component of the bacterial cell wall. The existing paradigm describes the peptidoglycan network as a static structure that is cross-linked predominantly by 4→3 transpeptide linkages. However, the nonclassical 3→3 linkages predominate the transpeptide networking of the peptidoglycan layer of nonreplicating Mycobacterium tuberculosis1,2. The molecular basis of these linkages and their role in the physiology of the peptidoglycan layer, virulence and susceptibility of M. tuberculosis to drugs remain undefined. Here we identify MT2594 as an L,D-transpeptidase that generates 3→3 linkages in M. tuberculosis. We show that the loss of this protein leads to altered colony morphology, loss of virulence and increased susceptibility to amoxicillin-clavulanate during the chronic phase of infection. This suggests that 3→3 cross-linking is vital to the physiology of the peptidoglycan layer. Although a functional homolog exists, expression of ldtMt2 is dominant throughout the growth phases of M. tuberculosis. 4→3 transpeptide linkages are targeted by one of the most widely used classes of antibacterial drugs in human clinical use today, β-lactams. Recently, meropenem-clavulanate was shown to be effective against drug-resistant M. tuberculosis3. Our study suggests that a combination of L,D-transpeptidase and β-lactamase inhibitors could effectively target persisting bacilli during the chronic phase of tuberculosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wietzerbin, J. et al. Occurrence of D-alanyl-(D)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of Mycobacteria. Biochemistry 13, 3471–3476 (1974).
Lavollay, M. et al. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation. J. Bacteriol. 190, 4360–4366 (2008).
Hugonnet, J.E., Tremblay, L.W., Boshoff, H.I., Barry, C.E. III & Blanchard, J.S. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323, 1215–1218 (2009).
Fauci, A.S. Multidrug-resistant and extensively drug-resistant tuberculosis: the National Institute of Allergy and Infectious Diseases Research agenda and recommendations for priority research. J. Infect. Dis. 197, 1493–1498 (2008).
Gandhi, N.R. et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368, 1575–1580 (2006).
Jindani, A., Dore, C.J. & Mitchison, D.A. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167, 1348–1354 (2003).
Wayne, L.G. & Sohaskey, C.D. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55, 139–163 (2001).
Betts, J.C., Lukey, P.T., Robb, L.C., McAdam, R.A. & Duncan, K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43, 717–731 (2002).
Voskuil, M.I. et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198, 705–713 (2003).
Keren, I., Shah, D., Spoering, A., Kaldalu, N. & Lewis, K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186, 8172–8180 (2004).
Goren, M.B. & Brennan, P.J. Tuberculosis (ed. Youmans, G.P.) 63 (W. B. Saunders, Philadelphia, 1979).
Vollmer, W. & Holtje, J.V. The architecture of the murein (peptidoglycan) in Gram-negative bacteria: vertical scaffold or horizontal layer(s)? J. Bacteriol. 186, 5978–5987 (2004).
Matsuhashi, M. [Biosynthesis in the bacterial cell wall] Tanpakushitsu Kakusan Koso 11, 875–886 (1966).
Lamichhane, G. et al. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 100, 7213–7218 (2003).
Mainardi, J.L. et al. A novel peptidoglycan cross-linking enzyme for a β-lactam–resistant transpeptidation pathway. J. Biol. Chem. 280, 38146–38152 (2005).
Lavollay, M. et al. The β-lactam-sensitive D,D-carboxypeptidase activity of Pbp4 controls the L,D and D,D transpeptidation pathways in Corynebacterium jeikeium. Mol. Microbiol. (in the press) (2009).
Hugonnet, J.E. & Blanchard, J.S. Irreversible inhibition of the Mycobacterium tuberculosis β-lactamase by clavulanate. Biochemistry 46, 11998–12004 (2007).
Donald, P.R. et al. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand. J. Infect. Dis. 33, 466–469 (2001).
Nadler, J.P., Berger, J., Nord, J.A., Cofsky, R. & Saxena, M. Amoxicillin–clavulanic acid for treating drug-resistant Mycobacterium tuberculosis. Chest 99, 1025–1026 (1991).
Ghuysen, J.M. Serine β-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 45, 37–67 (1991).
Waxman, D.J. & Strominger, J.L. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu. Rev. Biochem. 52, 825–869 (1983).
Crick, D.C. & Brennan, P.J. Biosynthesis of the arabinogalactan-peptidoglycan complex. in The Mycobacterial Cell Envelope (eds. Daffe, M. & Reyrat, J.) 25–40 (American Society for Microbiology, Washington, DC, 2008).
Templin, M.F., Ursinus, A. & Holtje, J.V. A defect in cell wall recycling triggers autolysis during the stationary growth phase of Escherichia coli. EMBO J. 18, 4108–4117 (1999).
Boneca, I.G. et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc. Natl. Acad. Sci. USA 104, 997–1002 (2007).
Lee, M.H., Pascopella, L., Jacobs, W.R. Jr. & Hatfull, G.F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis and bacille Calmette-Guerin. Proc. Natl. Acad. Sci. USA 88, 3111–3115 (1991).
Amrein, K.E. et al. Purification and characterization of recombinant human p50csk protein-tyrosine kinase from an Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc. Natl. Acad. Sci. USA 92, 1048–1052 (1995).
Auger, G., van Heijenoort, J., Mengin-Lecreulx, D. & Blanot, D.A. MurG assay which utilises a synthetic analogue of lipid I. FEMS Microbiol. Lett. 219, 115–119 (2003).
Arbeloa, A. et al. Synthesis of mosaic peptidoglycan cross-bridges by hybrid peptidoglycan assembly pathways in Gram-positive bacteria. J. Biol. Chem. 279, 41546–41556 (2004).
Wiegand, I., Hilpert, K. & Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008).
Acknowledgements
We gratefully acknowledge the support of US National Institutes of Health award AI30036. This work was also supported by the Foundation pour la Recherche Médicale (Equipe FRM 2006 (Dequation 200661107918)). M. Lavollay is the recipient of an Institut National de la Santé et de la Recherche Médicale PhD fellowship (Poste d'Accueil pour Pharmacien, Médecin, et Vétérinaire).
Author information
Authors and Affiliations
Contributions
R.G., W.R.B. and G.L. designed the project. J.-L.M. and M.A. designed the biochemical characterization of MT2594. M.L. and J.-L.M. performed biochemistry and analyzed data. R.G. and G.L. conducted genetics, microbiology and mouse experiments. G.L. wrote the manuscript with contributions from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–5 and Supplementary Methods (PDF 839 kb)
Rights and permissions
About this article
Cite this article
Gupta, R., Lavollay, M., Mainardi, JL. et al. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 16, 466–469 (2010). https://doi.org/10.1038/nm.2120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2120
This article is cited by
-
Peptidoglycan compositional analysis of Mycobacterium smegmatis using high-resolution LC–MS
Scientific Reports (2022)
-
Population Pharmacokinetics and Bayesian Dose Adjustment to Advance TDM of Anti-TB Drugs
Clinical Pharmacokinetics (2021)
-
Potential anti-TB investigational compounds and drugs with repurposing potential in TB therapy: a conspectus
Applied Microbiology and Biotechnology (2020)
-
Combination of Repurposed Drug Diosmin with Amoxicillin-Clavulanic acid Causes Synergistic Inhibition of Mycobacterial Growth
Scientific Reports (2019)
-
Identification of potent L,D-transpeptidase 5 inhibitors for Mycobacterium tuberculosis as potential anti-TB leads: virtual screening and molecular dynamics simulations
Journal of Molecular Modeling (2019)