Abstract
The analysis of rich catalogues of genetic variation from population-based sequencing provides an opportunity to screen for functional effects. Here we report a rare variant in APOC3 (rs138326449-A, minor allele frequency ~0.25% (UK)) associated with plasma triglyceride (TG) levels (−1.43 s.d. (s.e.=0.27 per minor allele (P-value=8.0 × 10−8)) discovered in 3,202 individuals with low read-depth, whole-genome sequence. We replicate this in 12,831 participants from five additional samples of Northern and Southern European origin (−1.0 s.d. (s.e.=0.173), P-value=7.32 × 10−9). This is consistent with an effect between 0.5 and 1.5 mmol l−1 dependent on population. We show that a single predicted splice donor variant is responsible for association signals and is independent of known common variants. Analyses suggest an independent relationship between rs138326449 and high-density lipoprotein (HDL) levels. This represents one of the first examples of a rare, large effect variant identified from whole-genome sequencing at a population scale.
Similar content being viewed by others
Introduction
Lipid levels are heritable risk factors for coronary artery disease, and vascular outcomes and their therapeutic manipulation has well-characterized impacts on disease risk1. Genome-wide studies of common genetic variation and its contribution to commonly measured lipid moieties have been successful in identifying a large number of associated loci2,3; however, the aggregate contribution of all of these confirmed common variants accounts currently for only about 10–12% of the variation in low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides (TGs)3. With current estimates of the heritability of these measures between 40% and 60%4, this leaves a considerable portion of variance unexplained. This may be contributed to by smaller common variant effects, as-yet undiscovered rare and potentially functional genetic variation, gene-by-gene interaction (epistasis) or by overestimates of heritability5. The availability of rich collections of variants through whole-genome sequencing (WGS) of well-phenotyped collections affords the unique opportunity to discover novel and potentially functional genetic variation associated with phenotypes of clinical interest.
Rare and highly penetrant variants identified in 24 different human genes have been identified through sequencing studies in families with rare monogenic lipid disorders6,7,8. Despite these studies, however, there has been little examination of these variants of low frequency (minor allele frequency (MAF)≤5%) on lipid profile at the level of the population. Studies that focus currently on exome content alone and have included variants of intermediate and low frequency have, however, reported larger genetic effects at lower MAF9,10,11. This type of work is relevant given that variants of large effect sizes have been suggested to segregate in populations at low frequencies under neutral or purifying models of evolution12. These genes and variants are likely to have considerable consequence on the health (expressed as odds ratios on the cardiovascular risk) for those who carry them, and may ultimately indicate novel therapeutic targets as already shown for PCSK9 (ref. 13).
The UK10K Cohorts project ( http://www.uk10k.org/studies/cohorts.html) uses WGS to study the contribution of low-frequency and rare variation on a broad range of complex quantitative endpoints. Here we applied low read-depth WGS in individuals from two deeply phenotyped British cohorts, TwinsUK14 and the Avon Longitudinal Study of Parents and Children (ALSPAC)15 to analyse TG levels. Analyses revealed replicable evidence of a rare, functional, variant in the APOC3 gene (rs138326449-A, MAF ~0.25% in the British population) strongly associated with plasma TG levels. This represents one of the first examples of a rare, large effect variant identified from WGS at a population based scale.
Results
Sequence data
A total of 3,910 individuals were sequenced to average 6.7 × mean read-depth using Illumina next-generation sequencing technology (Supplementary Methods ‘(Low read-depth WGS (cohorts data set)’). After applying stringent sample quality control filters, a total of 3,621 unrelated individuals of European ancestry (1,754 from TwinsUK and 1,867 from ALSPAC) were available for association. TG measurements were available for 3,202 individuals with sequence data, including 1,497 ALSPAC children (mean age 10 years, 50% females) and 1,705 TwinsUK adults, respectively (mean year 56 years, all females, Supplementary Table 1).
Phenotypic association
To search the human genome for low-frequency and rare variants associated with TG levels, we first tested associations with 13,074,236 single-nucleotide variants (SNV) and 1,122,542 biallelic indels (MAF ≥0.1%) called from whole-genome-sequence data (Supplementary Table 2). Associations of TGs with genetic variation were tested in the ALSPAC and TwinsUK WGS data sets separately, and study-specific summary statistics were combined using inverse variance meta-analysis (Methods). There was no evidence for inflation of summary statistics in the combined sample (λ(genomic control)=0.99, Supplementary Fig. 1).
No variants gave evidence of association at conventional levels of genome-wide significance. However, four variants reached a second more exploratory tier of evidence for discovery in tests of association with TG (P-value ≤1 × 10−7) across the UK10K sample and were of interest as they mapped to a region around the APOC3 locus on chromosome 11. Two of the variants found are common, rs964184-C (estimated allele frequency (EAF)=0.13%, P-value=6.81 × 10−9) and rs66505542-T (EAF=0.14%, P=1.87 × 10−8), in near-complete linkage disequilibrium (r2=0.90 in the UK10K sample) and have been previously associated with TG levels2,3.
A third association meeting the nominal discovery threshold is a novel variant with low MAF, also mapping to the APOC3 locus (Fig. 1). The rs138326449-A allele has an MAF of 0.25% and was associated with decreased TG levels corresponding to 1.43 (s.e=0.27) s.d. per allele (P-value=8.02 × 10−8) in the combined sample of 3,202 TwinsUK and ALSPAC participants with whole-genome sequence data (Fig. 2 and Table 1).
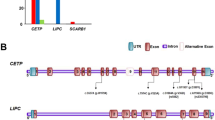
The figure is drawn using the UK10K Dalliance Browser. The tracks reported in a indicate (top to bottom): (i) P-value (on the –log10 scale) for association of SNPs in the APOC3 region with TG levels. Symbols are coloured corresponding to r2 to indicate the extent of linkage disequilibrium of each SNP in the region with the index SNPs rs964184 (red square) and the splice variant rs138326449 (blue triangle) marked; (ii) GENCODE genes (from ftp://ngs.sanger.ac.uk/production/gencode/). (b) A cartoon illustrating the genic location of rs138326449 in the context of variable splicing of the APOC3 gene.
Boxplots of associations between rs138326449 and TG, VLDL and HDL levels are shown as a function of carriage of allele A. Plots for HDL and TC are shown in Supplementary Fig. 2. P values indicate evidence for a linear relationship between lipid sub-fraction level and genotype (assuming an additive model). Box edges indicate the interquartile range (IQR; central line indicating the 50th centile) with the whisker indicating the lowest and highest daya still within 1.5 IQR of respective quartiles.
Signal validation and refinement
We first validated whole-genome sequence-derived rs138326449 genotypes using overlapping genotype calls and showed perfect concordance in both TwinsUK and ALSPAC (Methods). We then took forward the variant for replication in five additional cohorts (N=12,831; Supplementary Table 1), where the variant was imputed with high accuracy (defined by imputation info values ≥0.4) using a novel reference panel obtained from combining UK10K data with data from the 1000 Genomes Project (Supplementary Methods). The rs138326449-A allele had similar allele frequency in the five additional cohorts, with the highest value observed in a population isolate from Greece (MAF=0.8%; Supplementary Table 1). The five cohorts provided independent replication of the association with decreased TG (−1.0 (s.e.=0.173) s.d., P-value=7.32 × 10−9, combined discovery and replication P-value=6.92 × 10−15), suggesting that this variant contributes to decreasing TG levels in multiple populations of Northern and Southern European origin, with similar effect sizes and allelic frequency.
We further tested association of the splice variant with the other three main lipid sub-fractions HDL, LDL and total cholesterol (TC), and with very-low-density lipoprotein (VLDL). The A allele at rs138326449 was associated with decreased VLDL levels in a combined sample of 7,891 participants with available data (−1.312 (s.e.=0.199), P-value=4.16 × 10−11) and with a moderate increase in HDL in 16,062 study participants (0.624 (s.e.=0.143), P-value=1.36 × 10−5; Table 1 and Fig. 2). Associations with LDL and TC were negligible (Supplementary Table 3 and Supplementary Fig. 2).
Analysis of the residual association between rs138326449 and TG levels conditional on other lipid-sub-fractions showed expected patterns in both children and adults when taking into account lipid-lowering drugs. Adjustment for VLDL removed the association between rs138326449 and TG levels. In the children of the ALSPAC study, there was also evidence of association between rs138326449 and HDL levels after adjustment for either TG or VLDL, which was also seen (although less strongly) in the 1958 British Cohort (Table 2).
Further analysis of rare genetic variation
We next analysed the joint ALSPAC and TwinsUK sample with whole-genome sequence data to explore the contribution of common and rare genetic variants to TG associations in the APOC3 region. We focused on a 640-kb recombination window containing the novel signal at rs138326449. Association between rs138326449 and TG was assessed conditioning simultaneously on known associated variants best tagging all previously published common variant signals at this locus (rs964184 and rs2075290 (refs 2, 3)) and other potentially novel independent loci derived from region-specific conditional analysis in the UK10K data. Other than rs138326449 and positive controls from previous studies, the only other potential independent signal single-nucleotide polymorphism (SNP) in this region was rs193204541, and neither did conditional analysis, including this variant, abolish evidence for association at rs138326449 (Supplementary Table 4) nor was this particular signal supported using available replication data.
We also examined the potential additional contribution of variants (frequency at or below 1%) by using Sequence Kernel Association Testing (SKAT16) in ~3-kb windows tiled over the APOC3 region (Methods). Overall, seven windows had evidence for association with TG (P-value<1 × 10−3, equivalent to P-value=0.05 given a Bonferroni correction for multiple testing). The strongest of these was at chr11:116698501–116701500 (P-value=7.6 × 10−7). Despite specifically testing aggregates of rare variation, either one or a combination of the three independent SNP/SNV variants described above (that is, rs964184, rs2075290 and rs138326449) could account for six out of seven SKAT signals in this region (Supplementary Fig. 3). One region (chr11:116769001–116772000) showed nominal evidence of association with plasma TG levels (P-value=4 × 10−4) that could not be accounted for by association of any given individual SNV and that may represent a novel signal driven by multiple rare variants. Regional plots for all major lipid sub-fractions and SKAT results for this region can be found in Supplementary Figs 4 and 5). Results from a gene-based SKAT analysis across the APOC3 gave greatest evidence for association with APOC3 specifically; however, this region neither yielded results stronger than that shown from non-genic tiling nor were further regions implicated (Supplementary Table 5).
Variance explained
Overall, in UK10K data across ALSPAC and TwinsUK, genetic variation in the APOC3 region accounted for 2.71% (s.e.=1.39) of phenotypic variance in TG. This is in contrast to estimates of variance explained from the analysis of rs138326449 alone in children and adults not in the original discovery collections, which varied from 0.27 to 0.39% (Supplementary Table 6). Association results for known, TG-specific, positive controls are reported in Supplementary Table 7.
Discussion
Within the cohorts arm of the UK10K study, we have collected low read-depth, whole genome sequence data and used this with a validation and replication panel to describe a rare SNV (MAF ~0.2% in Europeans) strongly associated with plasma TG. The variant rs138326449 accounts for single point and sequence kernel-based association signals at the known Mendelian locus APOC3, independently of known associations at this locus. We have replicated the association in up to 12,852 study participants from 5 additional population samples of Northern and Southern European origin, confirming this association, albeit at a more modest level (difference in plasma TG levels −1.0 s.d. (s.e.=0.173) per minor allele). The rare allele association with plasma TG level is consistent with an effect of between 0.5 and 1.5 mmol l−1 across children and adults dependent on population (Supplementary Fig. 6a). This is considerably larger than that reported in recent examinations of common variation and is one of the first of this nature to be reported from the use of population-based WGS. In context, the largest reported lipid effects from existing genome-wide association study (GWAS) are up to five times greater than that for the commonly recognized FTOrs9939609 variant and adult body mass index (which is ~0.01 s.d. change in body mass index); however, these are still more than 20 times lower than that seen here2,17. It is also notable that this effect is found in both children and adults, and in the presence or absence of lipid-altering interventions (Supplementary Fig. 6b).
The human APOC3 gene is located in a gene cluster together with the APOA1 and APOA4 genes on the long arm of chromosome 11 (ref. 18). APOC3 is expressed in the liver and intestine, and is controlled by positive and negative regulatory elements that are spread throughout the gene cluster19,20,21. There is considerable evidence to support the genetic contribution of this locus to hyperlipidaemia and, in particular, there have been correlations between apoCIII levels, plasma TG and VLDL TGs22,23. With this, the use of fibrates as a therapeutic intervention (known to reduce the apoCIII synthesis rate in humans23) has suggested that there is an important role for APOC3 in TG metabolism. Moreover, transgenic mice expressing human apoCIII have shown that expression in the liver and intestine is correlated with elevated levels of VLDL TG, and where apoE is knocked out and APOC3 expressed, huge accumulations of TG-rich VLDL can occur24,25.
The splice donor site reported here lies in a region of chromosome 11 previously shown to contain both common and rare variants affecting plasma TG levels. Restriction fragment length polymorphism variation within the non-coding part of exon 4 at this locus, haplotypic characterization of variation in the region and a single change within exon 3 of APOC3 have all been related to either hypertriglyceridemia or familial combined hypercholesterolaemia26,27,28. More recently, a functional variant site (R19X) adjacent to rs138326449 and resulting in APOC3 loss-of-function in homozygote carriers has been reported independently in two genetic isolates from the United States and Greece; however, this variant is very rare (EAF=0.05%) in the general European population and does not contribute to variance in TG in this study29,30. In each case, the impaired expression of APOC3 is associated with reduced plasma TG levels and a coincident increase in HDL, in agreement with the inhibiting action of apoCIII on lipoprotein lipase. In the data here from UK10K, the total variance in plasma TGs explained by all genetic variation at this locus (down to and below 1% MAF) is ~2.7%. This is in comparison with this novel, low-frequency, genetic variant that accounts for somewhere between 0.27% and 0.39% of phenotypic variance.
The rs138326449 variant affects the essential di-nucleotide 5′-splicing site (GT to AT) of the first protein-coding exon of the protein-coding gene APOC3. The rare, TG-decreasing A allele is predicted to disrupt the correct splicing of the first protein-coding exon of APOC3 (containing the Apo-CIII domain (PF05778) and a signal peptide (1–20 aa)), resulting in a marked change of the 5′-splicing site score (from 4.37 (G) to −3.81 (A))31 (Supplementary Fig. 7). Although it was not possible to validate the splicing event using existing liver expression atlas generated by the GTex project32 because of the lack of carriers in this data set, we note that this position is highly conserved (phastCons=0.996, a measurement of evolutionary conservation based on multiple alignments of 100 vertebrates) through vertebrates33, supporting a probably potential, functional consequence for this site.
Recognized within the Adult Treatment Panel III and as part of the definition of the metabolic syndrome ( https://www.nhlbi.nih.gov/health-pro/guidelines/current/cholesterol-guidelines/index.htm), TG and TG-rich remnants are probably risk factors in cardiovascular disease34. Meta-analysis of 17 prospective studies has suggested that TGs are independent contributors to coronary heart disease risk and data from both the Münster Heart and Caerphilly studies have supported this35,36. This effect appears to be present independent of LDL-cholesterol and HDL-cholesterol levels37,38; however, these findings are not simple in interpretation. The current largest meta-analysis based on the same phenotypes has shown contradictory results39 and, in addition, an outstanding issue in these analyses remains the difficulty in assessing the independent impact of reduced or elevated TG levels from HDL. In our data, rs138326449 is associated with reduced TG in line with predicted lower levels of functional apoCIII in carriers of the A allele. However, this effect is not unique to TG levels, with a coincident and independent association with HDL making the interpretation of downstream effects of this variant (or variants at this locus exerting a similar effect) difficult in terms of causal inference.
In the absence of a clear explanation for the complex relationship between apolipoprotein gene effects and multiple lipid outcomes, the notion of overall lipid profile as a risk factor may be the most acceptable paradigm. To this end, variants such as rs138326449 do potentially provide information about the impact of interventions aimed at changing lipid profile. In this context, the impact of APOC3 inhibition through approaches such as targeted antisense oligonucleotide use40 can be modelled given observations such as that made in this study. This essentially represents an applied Mendelian randomization41 experiment, and with coincident disease status available, this type of study may help identify future, gene-targeted, therapeutic interventions.
Methods
ALSPAC WGS discovery sample
The ALSPAC is a long-term health research project. More than 14,000 mothers enrolled during pregnancy in 1991 and 1992, and the health and development of their children has been followed in great detail ever since. A random sample of 2,040 study participants was selected for WGS. The ALSPAC Executive Committee approved the study and all participants gave signed consent to the study.
Non-fasting plasma levels of TC, HDL and TG at age 9 years were measured with enzymatic colorimetric assays (Roche) on a Hitachi Modular P Analyser. HDL, TGs and TC (all in mmol l−1) were measured as described previously42. LDL was derived from the Friedwald formula: TC-(HDL Cholesterol+(TG/2.19))43. We calculated VLDL as VLDL Cholesterol (mmol l−1)=TC-LDL Cholesterol–HDL Cholesterol.
TwinsUK WGS discovery sample
TwinsUK is a nation-wide registry of volunteer twins in the United Kingdom, with about 12,000 registered twins (83% female, equal number of monozygotic and dizygotic twins, predominantly middle-aged and older). Over the last 20 years, questionnaire and blood/urine/tissue samples have been collected for over 7,000 subjects, as well as three comprehensive phenotyping assessments. The primary focus of study has been the genetic basis of healthy aging process and complex diseases, including cardiovascular, metabolic, musculoskeletal and ophthalmologic disorders. Alongside the detailed clinical, biochemical, behavioural and socio-economic characterization of the study population, the major strength of TwinsUK is availability of several ‘omics’ technologies for the participants. The database was used to study the genetic and environmental aetiology of age-related complex traits and diseases. The St Thomas’s Hospital Ethics Committee approved the study and all participants gave signed consent to the study.
Enzymatic colorimetric assays were used to measure serum levels of TC, HDL and TGs, and were measured using three analysing devices (Cobas Fara; Roche Diagnostics, Lewes, UK; Kodak Ektachem dry chemistry analysers (Johnson and Johnson Vitros Ektachem machine, Beckman LX20 analysers, Roche P800 modular system)). The majority of discovery samples were fasted before measurement (96%).
Low read-depth WGS (cohorts data set). Low read-depth WGS was performed at both the Wellcome Trust Sanger Institute and the Beijing Genomics Institute (BGI). DNA (1–3 μg) was sheared to 100–1,000 bp using a Covaris E210 or LE220 (Covaris, Woburn, MA, USA). Sheared DNA was subjected to Illumina paired-end DNA library preparation. Following size selection (300–500 bp insert size), DNA libraries were sequenced using the Illumina HiSeq platform as paired-end 100 base reads according to the manufacturer’s protocol.
Alignment and BAM processing
Data generated at the Sanger Institute and BGI were aligned to the human reference separately by the respective centres. The BAM files produced from these alignments were submitted to the European Genome-phenome Archive. The Vertebrate Resequencing Group at the Sanger Institute then performed further processing.
Alignment
Sequencing reads that failed quality control (QC) were removed using the Illumina GA Pipeline, and the rest were aligned to the GRCh37 human reference, specifically the reference used in Phase 1 of the 1000 Genomes Project ( ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/technical/reference/human_g1k_v37.fasta.gz). Reads were aligned using BWA (v0.5.9-r16)44.
BAM improvement and sample file production
Further processing to improve SNV and INDEL calling, including realignment around known INDELs, base quality score recalibration, addition of BAQ tags, merging and duplicate marking follows that used for Illumina low-coverage data in Phase 1 of the 1000 Genomes Project. Software versions used for UK10K for the steps described in that section were GATK version 1.1-5-g6f43284, Picard version 1.64 and samtools version 0.1.16.
Variant calling
SNV and INDEL calls were made using samtools/bcftools (version 0.1.18-r579)45 by pooling the alignments from 3,910 individual low read-depth BAM files. All-samples and all-sites genotype likelihood files (bcf) were created with samtools mpileup.
INDEL pre-filtering
The observation of spikes in the insertion/deletion ratio in sequencing cycles of a subset of the sequencing runs were linked to the appearance of bubbles in the flow cell during sequencing. To counteract this, the bamcheck utility from the samtools package was used to create a distribution of INDELs per sequencing cycle. Lanes with INDELs predominantly clustered at certain read cycles were marked as problematic (159 samples). In the next step, we checked mapped positions of the affected reads to see whether they overlapped with called INDELs, which they did for 1,694,630 called sites. The genotypes and genotype likelihoods of affected samples were then set to the reference genotype unless there was a support for the INDEL also in a different, unaffected lane from the same sample. In total, 140,163 genotypes were set back to reference and 135,647 sites were excluded by this procedure. Note that this step was carried out on raw, unfiltered calls before Variant Quality Score Recalibration filtering.
Site filtering
Variant Quality Score Recalibration46 was used to filter sites. For SNVs, the GATK (version 1.3–21) UnifiedGenotyper was used to recall the sites/alleles discovered by samtools to generate annotations to be used for recalibration. Recalibration for the INDELs used annotations derived from the built-in samtools annotations. The GATK VariantRecalibrator was then used to model the variants, followed by GATK ApplyRecalibration, which assigns VQSLOD (variant quality score log odds ratio) values to the variants. For SNV sites, a truth (GRCh37) sensitivity of 99.5%, which corresponded to a minimum VQSLOD score of −0.6804 was selected; that is, for this threshold, 99.5% of truth sites were retained. For INDEL sites, a truth sensitivity of 97%, which corresponded to a minimum VQSLOD score of 0.5939 was chosen. Finally, we also introduced the filter P<10−6 to remove sites that failed the Hardy–Weinberg equilibrium (302,388 sites removed) and removed sites with evidence for differential frequency (logistic regression P-value>1e−2) between samples sequenced at BGI and Wellcome Trust Sanger Institute (277,563 sites removed).
Given the presence of structure by genotyping batch, we ran a genome-wide association analysis for the binary variable ‘sequencing centre’ (‘BGI’/‘SANGER’) using a logistic regression model. SNPs (335,982) were associated with batch at a conservative threshold of P-value≤0.01 and formed a list that were subsequently filtered out from the genotype set, removing the batch effect due to sequencing centre.
Post-genotyping sample QC
Of the 4,030 samples (1,990 TwinsUK and 2,040 ALSPAC) that were submitted for sequencing, 3,910 samples (1,934 TwinsUK and 1,976 ALSPAC) were sequenced and went through the variant calling procedure. Low-quality samples were identified before the genotype refinement by comparing the samples with their GWAS genotypes using ~20,000 sites on chromosome 20 (see Supplementary Methods for full details).
Genotype refinement
The missing and low-confidence genotypes in the filtered VCFs were refined out through an imputation procedure with BEAGLE 4, rev909 (ref. 47). The programme was run with default parameters (see Supplementary Methods for full details). After imputation, chunks were recombined using the vcf-phased-join script from the vcftools [vcftools] package.
Post-refinement sample QC
Additional sample-level QC steps were carried out on refined genotypes, leading to the exclusion of additional 17 samples (16 TwinsUK and 1 ALSPAC) because of one or more of the following causes: (i) non-reference discordance with GWAS SNV data>5% (12 TwinsUK and 1 ALSPAC), (ii) multiple relations to other samples (13 TwinsUK and 1 ALSPAC) or (iii) failed sex check (3 TwinsUK and 0 ALSPAC).
To exclude the presence of participants of non-European ancestry in our data set, we merged a pruned data set to the 11 HapMap3 populations48 and performed a principal components analysis using EIGENSTRAT49. A total of 44 participants (12 TwinsUK and 32 ALSPAC) did not cluster to the European (CEU) cluster of samples and were removed from association analyses.
The final sequence data set that was used for the association analyses comprises 3,621 samples (1,754 TwinsUK and 1,867 ALSPAC).
Re-phasing
SHAPEIT2 (ref. 50) was then used to rephase the genotype data. The VCF files were converted to binary ped format. Multiallelic and MAF<0.02% (singleton and monomorphic) sites were removed. Files were then split into 3-mbp chunks with ±250 kbp flanking regions. SHAPEIT (v2.r727) was used to rephase the haplotypes.
Imputation from the combined UK10K+1000 Genomes Panel
For each of the cohorts, we had additional GWA data available. For ALSPAC, 6,557 samples were measured on Illumina HumanHap550 arrays and passed QC (population stratification, sex check, heterozygosity and relatedness (identity by state (IBS)>0.125)). For TwinsUK, 2,575 samples were genotyped on Illumina HumanHap300 or Illumina Human610 arrays. These samples passed QC on relatedness (IBS>0.125), population stratification, heterozygosity, zygosity and sex checks. Samples from the imputed data sets were unrelated to the sequence data sets (IBS>0.125). Variants discovered through WGS of the TwinsUK and ALSPAC cohorts were used for the development and use of a reference panel for imputation within the TwinsUK and ALSPAC GWA data sets. In other collections, these along with variants known from 1000 Genomes were imputed increasing the sample size for single point association analysis to 12,724 subjects. We developed new functionality in IMPUTE2 (ref. 51) that uses each reference panel to impute the missing variants in its counterpart, and then combine the two reference panels at the union set of sites. We tested the 3 reference panels for imputing 3 SNP array data, a sub-sample of 1,000 individuals from the UK10K WGS data set, 4 European samples (3 CEU, 1 TSI) sequenced by Complete Genomics (depth: 80 × )52 and an Italian isolate genotyped on core-exome SNP array (see Supplementary Methods for full details).
Validation genotyping
For ALSPAC, the entire cohort (10,145 participants, including 38 carriers of the rare A allele) was genotyped using KASP at KBioscience ( www.lgcgenomics.com/; see Supplementary Methods for full details). For TwinsUK, genotyping accuracy was evaluated against a data set comprising ~250 high-coverage exomes sequenced in overlapping samples53. Of the six carriers detected in our study, four were overlapping and correctly called also in the exome data set, yielding a genotyping accuracy of 100%. There was 100% concordance with the genotypes called from the whole-genome data set.
Trait standardization
Each cohort applied a standardized protocol for preparation of phenotypes, as follows. Female and male participants were divided into separate groups and TwinsUK participants were further divided into two unrelated subsets. Outliers deviating ≥4 or 5 s.d. (depending on the study) from the sample mean for a given trait were excluded from analysis (for this step, TGs were log transformed). The filtering of TG data by extremes of phenotype does not have a substantive impact on the numbers of rare variant carriers in this data set (although overall there is likely to be an enrichment for rare variant carriage in at the low end of the TG distribution in large collections). To approximate normality, each data set was inverse normal rank transformed in each group separately, and residuals were further computed by adjustment for age and age54 squared as a fixed effect. In TwinsUK, analyser effects were computed additionally as a random effect if associated with phenotype. Finally, residuals were standardized before combining males and females. In ALSPAC, trait residuals were computed jointly from the WGS and GWA samples. Details of trait transformation and statistical methods applied in each study are summarized in Supplementary Table 2. For conditional lipid analyses (Supplementary Table 4), all lipid sub-fractions and TC were inverse rank transformed before analyses. Pearson’s correlation coefficients were used to assess the correlation between variables, and linear regression was used to assess the relationship between variation at rs138326449 and lipid sub-fraction having serially conditioned on other lipids. For main analyses, where VLDL was missing from replication collections, it was not included given the correlation between TG and VLDL when derived from TC, HDL and LDL. This is illustrated where for regional analyses across ALSPAC and the 1958 British Cohort VLDL is calculated for purposes of illustration (Table 2).
Associations between lipids and SNVs and indels
We assessed associations between 14,196,778 genetic variants (13,074,236 SNPs and 1,122,542 biallelic indels, MAF ≥0.1%) and lipid traits (LDL, HDL, TG, TC and VLDL) calculated as described before, using linear regression models assuming additive genetic models. For primary analyses, associations were tested using a genotype dosage-based test implemented in the SNPTESTv4.2 software package54, apart for the TwinsUK GWAS and HELIC MANOLIS data sets, where mixed linear models were used to account for family structure using the GEMMA software55.
Meta-analysis of associations with SNVs
Summary statistics from individual studies were combined using fixed-effect inverse variance meta-analysis implemented in GWAMA v2.1 (ref. 56).
Region-specific analyses
Conditional analyses of genotype and rare variant aggregate association were undertaken within a joint sample from the UK10K cohort WGS data set (ALSPAC and TwinsUK) adjusting for study origin by residualizing transformed TG on an indicator variable for study. Records of region-specific recombination used to derive the recombination interval boundaries were retrieved from ( http://hapmap.ncbi.nlm.nih.gov/downloads/recombination/2011-01_phaseII_B37/), and this analysis was limited to a 640-kb window of chromosome 11 marked by a recombination fraction <25%. Evidence for previous genetic associations was available from existing studies2,3 and best tag variants for positive controls were derived by using PLINK to assess linkage disequilibrium across positive controls57. Evidence for further novel independent TG associations across the APOC3 region in this data set were assessed using GCTA58. We considered all SNPs and bi-allelic indels seen at least twice that had any evidence of association with TG (P<1 × 10−3) alongside those previously associated with lipid levels irrespective of association result in this sample58,60. Conditional analyses were undertaken for rs138326449 given all potentially independent contributing loci in this region using GCTA. GCTA was also used to calculate the total genetic contribution to variance in TG for the same region having calculated a matric of relatedness from the whole of chromosome 11. In addition to regional analyses, estimates of variance explained for rs138326449 alone were derived from the ALSPAC and 1958 birth cohort collections using linear regression taking into account the covariables age, age, sex and lipid-lowering drugs in the case of the 1958 birth cohort. Analyses for this were undertaken using STATA version 13 (StataCorp. 2013. Stata Statistical Software: Release 13; StataCorp LP, College Station, TX).
SKAT16 was undertaken across the APOC3 region. Sequence-derived genotypes with MAF capped at 1% were extracted and split into sub-regions containing as close to 50 variants as possible. These were analysed using SKAT and all signals with evidence for association P-value<1 × 10−3, equivalent to P-value=0.05 given a Bonferroni correction for multiple testing across this region, were taken forward for further analyses. We then re-formulated phenotype-containing fam files for SKAT analysis having conditioned sequentially on known positive control or novel independent contributing SNPs in the region before re-running SKAT analyses. Results from a gene-based SKAT analysis were generated by running SKAT (again with MAF ≤1%) for genes contained within the APOC3 region. Genes for this analysis were defined by GENCODE (v15) within positions 115,820,914 and 117,103,241 on chromosome 11. In more detail, variants within exons and splice variants were tested in windows up to 51 variants per window. If there were >50 exonic and splice variants per gene, then variants were split in two ways: first by combining neighbouring exons so that the number of variants was about evenly split between windows, and second by tiling across the concatenated exons with maximal 51 variants per window but starting halfway the first window that was generated by the first approach.
Replication samples
Description of the replication samples is given in the Supplementary Methods.
Disclaimer
This publication is the work of the authors and N.J.T., G.D.S. and S.R. will serve as guarantors for the contents of this paper. N.J.T. and G.D.S. work within a MRC unit at the University of Bristol. Please note that the ALSPAC website contains details of all the data that is available through a fully searchable data dictionary ( www.bris.ac.uk/alspac/researchers/data-access/data-dictionary). T.D.S. is holder of an ERC Advanced Principal Investigator award.
Additional information
How to cite this article: Timpson, N. J. et al. A rare variant in APOC3 is associated with plasma triglyceride and VLDL levels in Europeans. Nat. Commun. 5:4871 doi: 10.1038/ncomms5871 (2014).
Change history
12 May 2015
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Arsenault, B. J., Boekholdt, S. M. & Kastelein, J. J. Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 8, 197–206 (2011).
Global Lipids Genetics, C.. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013).
Teslovich, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010).
Weiss, L. A., Pan, L., Abney, M. & Ober, C. The sex-specific genetic architecture of quantitative traits in humans. Nat. Genet. 38, 218–222 (2006).
Zuk, O., Hechter, E., Sunyaev, S. R. & Lander, E. S. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc. Natl Acad. Sci. 109, 1193–1198 (2012).
Johansen, C. T. et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 42, 684–687 (2010).
Cohen, J. et al. Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science (New York, NY) 305, 869–872 (2004).
Stitziel, N. O. et al. Exome sequencing and directed clinical phenotyping diagnose cholesterol ester storage disease presenting as autosomal recessive hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 33, 2909–2914 (2013).
Lange, L. A. et al. Whole-exome sequencing identifies rare and low-frequency coding variants associated with LDL cholesterol. Am. J. Hum. Genet. 94, 233–245 (2014).
Peloso, Gina M. et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 Whites and Blacks. Am. J. Hum. Genet. 94, 223–232 (2014).
Holmen, O. L. et al. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat. Genet. 46, 345–351 (2014).
Goldstein, D. B. et al. Sequencing studies in human genetics: design and interpretation. Nat. Rev. Genet. 14, 460–470 (2013).
Kotowski, I. K. et al. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 78, 410–422 (2006).
Moayyeri, A., Hammond, C. J., Valdes, A. M. & Spector, T. D. Cohort Profile: TwinsUK and healthy ageing twin study. Int. J. Epidemiol. 42, 76–85 (2013).
Boyd, A. et al. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 42, 111–127 (2013).
Wu, M. C. et al. Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 89, 82–93 (2011).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Karathanasis, S. K. Apolipoprotein multigene family: tandem organization of human apolipoprotein AI, CIII, and AIV genes. Proc. Natl Acad. Sci. USA 82, 6374–6378 (1985).
Reue, K., Leff, T. & Breslow, J. L. Human apolipoprotein CIII gene expression is regulated by positive and negative cis-acting elements and tissue-specific protein factors. J. Biol. Chem. 263, 6857–6864 (1988).
Ogami, K., Hadzopoulou-Cladaras, M., Cladaras, C. & Zannis, V. I. Promoter elements and factors required for hepatic and intestinal transcription of the human ApoCIII gene. J. Biol. Chem. 265, 9808–9815 (1990).
Vergnes, L., Taniguchi, T., Omori, K., Zakin, M. M. & Ochoa, A. The apolipoprotein A-I/C-III/A-IV gene cluster: ApoC-III and ApoA-IV expression is regulated by two common enhancers. Biochim. Biophys. Acta. 1348, 299–310 (1997).
Carlson, L. A. & Ballantyne, D. Changing relative proportions of apolipoproteins CII and CIII of very low density lipoproteins in hypertriglyceridaemia. Atherosclerosis 23, 563–568 (1976).
Malmendier, C. L. et al. Apolipoproteins C-II and C-III metabolism in hypertriglyceridemic patients. Effect of a drastic triglyceride reduction by combined diet restriction and fenofibrate administration. Atherosclerosis 77, 139–149 (1989).
Aalto-Setala, K. et al. Further characterization of the metabolic properties of triglyceride-rich lipoproteins from human and mouse apoC-III transgenic mice. J. Lipid Res. 37, 1802–1811 (1996).
Ebara, T., Ramakrishnan, R., Steiner, G. & Shachter, N. S. Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J. Clin. Invest. 99, 2672–2681 (1997).
Dallinga-Thie, G. M. et al. Complex genetic contribution of the Apo AI-CIII-AIV gene cluster to familial combined hyperlipidemia. Identification of different susceptibility haplotypes. J. Clin. Invest. 99, 953–961 (1997).
Dallinga-Thie, G. M. et al. Apolipoprotein A-I/C-III/A-IV gene cluster in familial combined hyperlipidemia: effects on LDL-cholesterol and apolipoproteins B and C-III. J. Lipid Res. 37, 136–147 (1996).
Ribalta, J. et al. A variation in the apolipoprotein C-III gene is associated with an increased number of circulating VLDL and IDL particles in familial combined hyperlipidemia. J. Lipid Res. 38, 1061–1069 (1997).
Tachmazidou, I. et al. A rare functional cardioprotective APOC3 variant has risen in frequency in distinct population isolates. Nat. Commun. 4, 2872 (2013).
Pollin, T. I. et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 322, 1702–1705 (2008).
Yeo, G. & Burge, C. B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 11, 377–394 (2004).
Consortium, G. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Siepel, A. et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15, 1034–1050 (2005).
Varbo, A., Benn, M. & Nordestgaard, B. G. Remnant cholesterol as a cause of ischemic heart disease: Evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol. Ther. 141, 358–367 (2014).
Hokanson, J. E. & Austin, M. A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk 3, 213–219 (1996).
Yarnell, J. W. et al. Do total and high density lipoprotein cholesterol and triglycerides act independently in the prediction of ischemic heart disease? Ten-year follow-up of Caerphilly and Speedwell Cohorts. Arterioscler. Thromb. Vasc. Biol. 21, 1340–1345 (2001).
Varbo, A., Benn, M., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 128, 1298–1309 (2013).
Do, R. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
The Emerging Risk Factors, C. MAjor lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Visser, M. E., Witztum, J. L., Stroes, E. S. & Kastelein, J. J. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur. Heart. J. 33, 1451–1458 (2012).
Davey Smith, G. & Ebrahim, S. Mendelian randomization: prospects, potentials, and limitations. Int. J. Epidemiol. 33, 30–42 (2004).
Williams, D. M. et al. Associations of maternal 25-hydroxyvitamin D in pregnancy with offspring cardiovascular risk factors in childhood and adolescence: findings from the Avon Longitudinal Study of Parents and Children. Heart. 99, 1849–1856 (2013).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Browning, S. R. & Browning, B. L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097 (2007).
International HapMap, C.. et al. Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
O'Connell, J. et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 10, e1004234 (2014).
Howie, B. N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Drmanac, R. et al. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science 327, 78–81 (2010).
Williams, F. M. et al. Genes contributing to pain sensitivity in the normal population: an exome sequencing study. PLoS Genet. 8, e1003095 (2012).
Marchini, J. & Howie, B. Genotype imputation for genome-wide association studies. Nat. Rev. Genet. 11, 499–511 (2010).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Magi, R. & Morris, A. P. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 11, 288 (2010).
Purcell, S. et al. PLINK: A tool for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380, 572–580 (2012).
Protter, A. A. et al. Isolation and sequence analysis of the human apolipoprotein CIII gene and the intergenic region between the apo AI and apo CIII genes. DNA 3, 449–456 (1984).
Acknowledgements
This study makes use of data generated by the UK10K Consortium, derived from samples from the ALSPAC and TwinsUK data sets. A full list of the investigators who contributed to the generation of the data is available from www.UK10K.org. Funding for UK10K was provided by the Wellcome Trust under award WT091310. This work made use of data and samples generated by the 1958 Birth Cohort (NCDS). Access to these resources was enabled via the 58READIE Project funded by Wellcome Trust and Medical Research Council (grant numbers WT095219MA and G1001799). A full list of the financial, institutional and personal contributions to the development of the 1958 Birth Cohort Biomedical resource is available at www2.le.ac.uk/projects/birthcohort. Genotyping was undertaken as part of the Wellcome Trust Case-Control Consortium (WTCCC) under Wellcome Trust award 076113, and a full list of the investigators who contributed to the generation of the data is available at www.wtccc.org.uk. We also are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. TwinsUK was funded by the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007-2013). The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King’s College London. SNP Genotyping was performed by The Wellcome Trust Sanger Institute and National Eye Institute via NIH/CIDR. S.S. is supported by an Oak Foundation Research Fellowship. N.S. is supported by the Wellcome Trust (Grant Codes WT098051 and WT091310), the EU FP7 (EPIGENESYS Grant Code 257082 and BLUEPRINT Grant Code HEALTH-F5-2011-282510). This work was also supported by the Wellcome Trust (098051) and the European Research Council (ERC-2011-StG 280559-SEPI). We are grateful to the residents of the Pomak villages and of the Mylopotamos villages for taking part in the HELIC study, and to Echinos Medical Centre and Anogia Medical Centre for their contribution to the collection. S.E.H. and M.F. are supported by the British Heart Foundation (PG008/08). NJT and GDS work within an MRC Unit at the University of Bristol (MC_UU_12013/1–9).
Author information
Authors and Affiliations
Consortia
Contributions
Manuscript preparation: N.S. and N.J.T. Data analysis: N.J.T., K.W., J.L.M., I.T., G.M., M.C., S.-Y.S., L.C., L.S., V.I., J.H., S.Mc.C., P.D. and R.D. Provision of data and materials: T.D.S., G.D.S., M.F., S.E.H., G.G., J.B.R., D.M., S.M.R., A.G., G.D., P.B., P.J.T. and E.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-7, Supplementary Tables 1-7, Supplementary Methods and Supplementary References (PDF 3155 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Timpson, N., Walter, K., Min, J. et al. A rare variant in APOC3 is associated with plasma triglyceride and VLDL levels in Europeans. Nat Commun 5, 4871 (2014). https://doi.org/10.1038/ncomms5871
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms5871
This article is cited by
-
CRISPR/Cas9-mediated knockout of APOC3 stabilizes plasma lipids and inhibits atherosclerosis in rabbits
Lipids in Health and Disease (2021)
-
Metabolic characterisation of disturbances in the APOC3/triglyceride-rich lipoprotein pathway through sample-based recall by genotype
Metabolomics (2020)
-
Frequency and phenotype consequence of APOC3 rare variants in patients with very low triglyceride levels
BMC Medical Genomics (2018)
-
Genetic architecture: the shape of the genetic contribution to human traits and disease
Nature Reviews Genetics (2018)
-
Hepatitis B virus inhibits the in vivo and in vitro synthesis and secretion of apolipoprotein C3
Lipids in Health and Disease (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.