Abstract
Porous solids have an important role in addressing some of the major energy-related problems facing society. Here we describe a porous solid, α-MnO2, with a hierarchical tetramodal pore size distribution spanning the micro-, meso- and macro pore range, centred at 0.48, 4.0, 18 and 70 nm. The hierarchical tetramodal structure is generated by the presence of potassium ions in the precursor solution within the channels of the porous silica template; the size of the potassium ion templates the microporosity of α-MnO2, whereas their reactivity with silica leads to larger mesopores and macroporosity, without destroying the mesostructure of the template. The hierarchical tetramodal pore size distribution influences the properties of α-MnO2 as a cathode in lithium batteries and as a catalyst, changing the behaviour, compared with its counterparts with only micropores or bimodal micro/mesopores. The approach has been extended to the preparation of LiMn2O4 with a hierarchical pore structure.
Similar content being viewed by others
Introduction
Porous solids have an important role in addressing some of the major problems facing society in the twenty-first century, such as energy storage, CO2 sequestration, H2 storage, therapeutics (for example, drug delivery) and catalysis1,2,3,4,5,6,7,8. The size of the pores and their distribution directly affect their ability to function in a particular application2. For example, zeolites are used as acid catalysts in industry, but their micropores impose severe diffusion limitations on the ingress and egress of the reactants and the catalysed products9. To address such issues, great effort is being expended in preparing porous materials with a bimodal (micro and meso) pore structure by synthesizing zeolites or silicas containing micropores and mesopores10,11,12,13,14,15,16,17, or microporous metal–organic frameworks with ordered mesopores18. Among porous solids, porous transition metal oxides are particularly important, because they exhibit many unique properties due to their d-electrons and the variable redox state of their internal surfaces8,19,20,21,22.
Here we describe the first solid (α-MnO2) possessing hierarchical pores spanning the micro, meso and macro range, centred at 0.48, 4.0, 18 and 70 nm. The synthesis method uses mesoporous silica as a hard template. Normally such a template generates a mesoporous solid with a unimodal23,24,25,26,27,28,29,30,31 or, at most, a bimodal pore size distribution32,33,34,35,36,37,38. By incorporating K+ ions in the precursor solution, within the silica template, the K+ ions act bifunctionally: their size templates the formation of the micropores in α-MnO2, whereas their reactivity with silica destroys the microporous channels in KIT-6 comprehensively, leading to the formation of α-MnO2 containing large mesopores and, importantly, macropores, something that has not been possible by other methods. Significantly, this is achieved without destroying the silica template by alkaline ions. The effect of the tetramodal pore structure on the properties of the material is exemplified by considering their use as electrodes for lithium-ion batteries and as a catalyst for CO oxidation and N2O decomposition. The novel material offers new possibilities for combining the selectivity of small pores with the transport advantages of the large pores across a wide range of sizes. We also present results demonstrating the extension of the method to the synthesis of LiMn2O4 with a hierarchical pore structure.
Results
Composition of tetramodal α-MnO2
The composition of the synthesized material was determined by atomic absorption analysis and redox titration to be K0.08MnO2 (the K/Mn ratio of the precursor solution was 1/3). The material is commonly referred to as α-MnO2, because of the small content of K+19.
N2 sorption analysis of tetramodal α-MnO2
The tetramodal α-MnO2 shows a type IV isotherm (Fig. 1a). The pore size distribution (Fig. 1b) in the range of 0.3–200 nm was analysed using the density functional theory (DFT) method applied to the adsorption branch of the isotherm39,40,41,42, as this is more reliable than analysing the desorption branch43; note that this is not the DFT method used in ab initio electronic structure calculations. Plots were constructed with vertical axes representing ‘incremental pore volume’ and ‘incremental surface area’. Large (macro) pores can account for a significant pore volume while representing a relatively smaller surface area and vice versa for small (micro) pores. Therefore, when investigating a porous material with a wide range of pore sizes, for example, micropore and macropore, the combination of surface area and pore volume is essential to determine the pore size distribution satisfactorily (Fig. 1b). Considering both pore volume and surface area, significant proportions of micro-, meso- and macropores are evident, with distinct maxima centred at 0.70, 4.0, 18 and 70 nm. To probe the size of the micropores more precisely than is possible with DFT, the Horvath–Kawazoe pore size distribution analysis was employed44. A single peak was obtained at 0.48 nm (Fig. 1c), in good accord with the 0.46-nm size of the 2 × 2 channels of α-MnO2 (refs. 19, 21). The relatively small Brunauer–Emmett–Teller (BET) surface area of tetramodal α-MnO2 (79–105 m2 g−1; Supplementary Table S1) compared with typical surface areas of mesoporous metal oxides (90–150 m2 g−1)45 is due to the significant proportion of macropores (which have small surface areas) and relatively large (18 nm) mesopores—a typical mesoporous metal oxide has only 3–4 nm pores.
TEM analysis of tetramodal α-MnO2
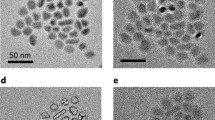
Transmission electron microscopic (TEM) data for tetramodal α-MnO2, Fig. 2, demonstrates a three-dimensional pore structure with a symmetry consistent with space group Ia3d. From the TEM data, an a0 lattice parameter of 23.0 nm for the mesostructure could be extracted, which is in good agreement with the value obtained from the low-angle powder X-ray diffraction (PXRD) data, a0=23.4 nm (Supplementary Fig. S1a). High-resolution TEM images in Fig. 2c–e demonstrate that the walls are crystalline with a typical wall thickness of 10 nm. The lattice spacings of 0.69, 0.31 and 0.35 nm agree well with the values of 6.92, 3.09 and 3.46 Å for the [110], [310] and [220] planes of α-MnO2 (International Centre for Diffraction Data (ICDD) number 00-044-0141), respectively. The wide-angle PXRD data matches well with the PXRD data of bulk cryptomelane α-MnO2 (Supplementary Fig. S1b), confirming the crystalline walls.
TEM images along (a) [100] direction, showing 18 nm mesopores (scale bar, 50 nm); (b) 4.0 and 70 nm pores (70 nm pores are highlighted by white circles; scale bar, 100 nm); (c–e) high-resolution (HRTEM) images of tetramodal α-MnO2 showing 4.0 and 0.48 nm pores (scale bar, 10 nm). Inset is representation of α-MnO2 structure along the c axis, demonstrating the 2 × 2 micropores as shown in the HRTEM (white box) in e. Purple,octahedral MnO6; red, oxygen; violet, potassium.
The various pores in tetramodal α-MnO2 can be observed by TEM directly: the 0.48-nm micropores are seen in Fig. 2e (2 × 2 tunnels with dimensions of 0.48 × 0.48 nm in the white box); the 4.0-nm pores are shown in Fig. 2b–d; the 18-nm pores are shown in Fig. 2a; the 70-nm pores are evident in Fig. 2b (highlighted with white circles).
Li intercalation
Li can be intercalated into bulk α-MnO2 (ref. 46). Therefore, it is interesting to compare Li intercalation into bulk α-MnO2 (micropores only) and bimodal α-MnO2 (micropores along with a single mesopore of diameter 3.6 nm, see Methods) with tetramodal α-MnO2 (micro-, meso- and macropores). Each of the three α-MnO2 materials was subjected to Li intercalation by incorporation as the positive electrode in a lithium battery, along with a lithium anode and a non-aqueous electrolyte (see Methods). The results of cycling (repeated intercalation/deintercalation of Li) the cells are shown in Fig. 3. Although all exhibit good capacity to cycle Li at low rates of charge/discharge (30 mA g−1), tetramodal α-MnO2 shows significantly higher capacity (Li storage) at a high rate of 6,000 mA g−1 (corresponding to charge and discharge in 3 min). The tetramodal α-MnO2 can store three times the capacity (Li) compared with bimodal α-MnO2, and 18 times that of α-MnO2 with only micropores, at the high rate of intercalation/deintercalation (Fig. 3). The superior rate capability of tetramodal α-MnO2 over microporous and bimodal forms may be assigned to better Li+ transport in the electrolyte within the hierarchical pore structure of tetramodal α-MnO2. The importance of electrolyte transport in porous electrodes has been discussed recently35,47,48 and the results presented here reinforce the beneficial effect of a hierarchical pore structure.
Catalytic studies
CO oxidation and N2O decomposition were used as reactions to probe the three different forms of α-MnO2 as catalysts (Supplementary Fig. S2). As shown in Supplementary Fig. S2a, tetramodal α-MnO2 demonstrates better catalytic activity compared with only micropores or bimodal α-MnO2; the temperature of half CO conversion (T50) was 124 °C for tetramodal α-MnO2, whereas microporous and bimodal α-MnO2 exhibited a T50 value of 275 °C and 209 °C, respectively. In the case of N2O decomposition, α-MnO2 with only micropores demonstrated no catalytic activity in the range of 200–400 °C, in accord with a previous report49. Tetramodal and bimodal α-MnO2 showed catalytic activity and reached 32% and 20% of N2O conversion, respectively, at a reaction temperature of 400 °C. The differences in catalytic activity are related to the differences in the material. A detailed study focusing on the catalytic activity alone would be necessary to demonstrate which specific features of the textural differences (pore size distribution, average manganese oxidation state, K+ and so on) between the different MnO2 materials are responsible for the differences in behaviour. However, the preliminary results shown here do illustrate that such differences exist.
Porous LiMn2O4
To demonstrate the wider applicability of the synthesis method, LiMn2O4 with a hierarchical pore structure was synthesized in a way similar to that of tetramodal α-MnO2. The main difference is the use of LiNO3 instead of KNO3 (see Methods). In this case, Li+ reacts with the silica template collapsing/blocking the microporous channels in the KIT-6 and resulting in the large mesopores and macropores (17 and 50 nm) in the LiMn2O4 obtained. The use of Li+ instead of the larger K+ deters the formation of micropores because Li+ is too small.
TEM analysis illustrates the hierarchical pore structure of LiMn2O4 (Supplementary Fig. S3): 4.0 nm pores are evident in Supplementary Fig. S3b; 17 nm pores in Supplementary Fig. S3a; and 50 nm pores in Supplementary Fig. S3b (highlighted with white circles). The d-spacing of 0.47 nm in the high-resolution TEM image (Supplementary Fig. S3c) is in good accordance with the values of 0.4655, nm for the [111] planes of LiMn2O4 (ICDD number 00-038-0789) and with the wide-angle PXRD data (Supplementary Fig. S4). The original DFT pore size distribution analysis from N2 sorption (adsorption branch) gives three pore sizes in the range of 1–100 nm centred at 4.0, 17 and 50 nm (Supplementary Fig. S5). A more in-depth presentation of the results for LiMn2O4 will be given in a future paper; preliminary results presented here illustrate that the basic method can be applied beyond α-MnO2.
Discussion
Turning to the synthesis of the tetramodal α-MnO2, the details are given in the Methods section. Hard templating using silica templates, such as KIT-6, normally gives rise to materials with unimodal or, at most, bimodal mesopore structures, and in the latter case the smaller mesopores dominate over the larger mesopores8,32,35. Alkali ions are excellent templates for micropores in transition metal oxides19,21, but they have been avoided in nanocasting from silica templates because of concerns that they would react with and, hence, destroy the silica template50. Here, not only have alkali ions been used successfully in precursor solutions without destroying the template mesostructure but they give rise to macropores in the α-MnO2, thus permitting the synthesis of a tetramodal, micro-small, meso-large, meso-macro pore structure.
Synthesis begins by impregnating the KIT-6 silica template with a precursor solution containing Mn2+ and K+ ions. On heating, the K+ ions template the formation of the micropores in α-MnO2, as the latter forms within the KIT-6 template. KIT-6 consists of two interpenetrating mesoporous channels linked by microporous channels51,52,53. The branches of the two different sets of mesoporous channels in KIT-6 are nearest neighbours separated by a silica wall of ~4 nm53; therefore, when both KIT-6 mesochannels are occupied by α-MnO2 and the silica between them etched away, the remaining pore is 4 nm (see centre portion of Fig. 4). It has been shown previously, by a number of authors, that by varying the hydrothermal conditions used to prepare the KIT-6, the proportion of the microchannels can be decreased to some extent, thus making it difficult to simultaneously fill the neighbouring KIT-6 mesoporous channels by the precursor solution of the target mesoporous metal oxide33,34,35. As a result, the target metal oxide grows in only one set of mesochannels of the KIT-6 host but not both. When the KIT-6 is dissolved away, the remaining metal oxide has ~18 nm pores, because the distance between adjacent branches of the same KIT-6 mesochannels is greater than between the two different mesochannels in KIT-6. Here we propose that the K+ ions have a similar effect on the KIT-6 to that of the hydrothermal synthesis, but by a completely different mechanism. Reaction between the K+ ions in the precursor solution with the silica during calcination results in the formation of K+-silicates, which cause collapse or blocking of the microporous channels in KIT-6, such that the α-MnO2 grows in one set of the KIT-6 mesochannels, giving rise to 18 nm pores in α-MnO2 when the silica is etched away, see top portion of Fig. 4. However, the reaction between K+ and the silica is more severe than the effect of varying the hydrothermal treatment. In the former case, the KIT-6 microchannels are so comprehensively destroyed that the proportion of the large (18 nm) to smaller (4 nm) mesopores is greater than can be achieved by varying hydrothermal conditions. The comprehensive destruction of the microchannels in KIT-6 by K+, perhaps augmented by some minor degradation of parts of the mesochannels, leads to α-MnO2 growing in only a proportion of one set of the KIT-6 mesochannels, resulting in the formation of ~70 nm pores in α-MnO2, see lower portion of Fig. 4. In summary, the K+ reactivity with the silica goes beyond what can be achieved by varying the conditions of hydrothermal synthesis and is responsible for generating the tetramodal pore size distribution reported here.
When both KIT-6 mesochannels are occupied by α-MnO2 and then the silica between them etched away, the remaining pore is 4 nm (centre portion of figure). When α-MnO2 grows in only one set of mesochannels and then the KIT-6 is dissolved away, the remaining metal oxide has 18 nm pores (upper portion of figure). The comprehensive destruction of the microchannels in KIT-6 by K+ leads to α-MnO2 growing in only a proportion of one set of the KIT-6 mesochannels, resulting in the formation of ~70 nm pores (lower portion of figure).
The mechanism of pore formation in α-MnO2 by reaction between K+ and the silica template is supported by several findings. First, by the lower K/Mn molar ratio of the final tetramodal α-MnO2 product (0.08) compared with the starting materials (0.33) implies that some of the K+ ions in the impregnating solution have reacted with the silica. Second, support for collapse/blocking of the microporous channels in KIT-6 due to reaction with K+ was obtained by comparing the texture of KIT-6 impregnated with an aqueous solution containing only KNO3 and calcined at 300 and 500 °C. The micropore volume in KIT-6 is the greatest, with no KNO3 in the solution; it then decreases continuously as the calcination temperature and calcination time is increased, such that after 2 and 5 h at 500 °C the micropore volume has decreased to zero (Supplementary Fig. S6). Third, we prepared tetramodal α-MnO2 using a similar synthetic procedure to that described in the Methods section, except that this time we used a covered tall crucible for the calcination step. Sun et al.54 have shown that using a covered, tall crucible when calcining results in porous metal oxides with much larger particle sizes. If the 70-nm pores had arisen simply from the gaps between the particles, then the pore size would have changed; in contrast, it remained centred at 70 nm, Supplementary Fig. S7, consistent with the 70-nm pores being intrinsic to the materials and arising from reaction with the K+ as described above. Fourth, if the synthesis of MnO2 is carried out using the KIT-6 template but in the absence K+ ions, then the DFT pore size distribution shown in Supplementary Fig. S8 is obtained. The 0.48- and 70-nm pores are now absent, but the 4- and 18-nm pores remain. This demonstrates the key role of K+ in the formation of the smallest and largest pores and, hence, in generating the tetramodal pore size distribution. The absence of K+ means that there is nothing to template the 0.48 nm pores and so α-MnO2 is not formed; the β-polymorph is obtained instead. The absence of K+ also means that the microchannels in the KIT-6 template remain intact, resulting in no 70 nm pores and the dominance of the 4-nm pores compared with the 18-nm pores.
The hierarchical pore structure can be varied systematically by controlling the synthesis conditions, in particular the K+/Mn ratio of the precursor solution. A range of K+/Mn ratios, 1/5, 1/3 and 1/2, gave rise to a series of pore size distributions, in which the pore sizes remained the same but the relative proportions of the different pores varied (Supplementary Table S1). The higher the K+/Mn ratio, the greater the proportion of macropores and large mesopores. This is in accord with expectations, as the higher the K+ concentration in the precursor solution the greater the collapse/blocking of the microporous channels in the KIT-6 (as noted above), and hence the greater the proportion of macropores and large mesopores. Indeed, these results offer further support for the mechanism of pore size distribution arising from reaction between K+ and the silica template.
In conclusion, tetramodal α-MnO2, the first porous solid with a tetramodal pore size distribution, has been synthesized. Its hierarchical pore structure spans the micro, meso and macropore range between 0.3 and 200 nm, with pore dimensions centred at 0.48, 4.0, 18 and 70 nm. Key to the synthesis is the use of K+ ions that not only template the formation of micropores but also react with the silica template, therefore, breaking/blocking the microporous channels in the silica template far more comprehensively than is possible by varying the hydrothermal synthesis conditions, to the extent that macropores are formed, and without destroying the silica mesostructure by alkali ions, as might have been expected. The resulting hierarchical tetramodal structure demonstrates different behaviours compared with microporous and bimodal α-MnO2 as a cathode material for Li-ion batteries, and when used as a catalyst for CO oxidation and N2O decomposition. The method has been extended successfully to the preparation of hierarchical LiMn2O4.
Methods
Synthesis
Tetramodal α-MnO2 (surface area 96 m2 g−1, K0.08MnO2) was prepared by two-solvent impregnation55 using K+ and mesoporous silica KIT-6 as the hard template. KIT-6 was prepared according to a previous report (hydrothermal treatment at 100 °C)51. In a typical synthesis of tetramodal α-MnO2, 7.53 g of Mn(NO3)2·4H2O (98%, Aldrich) and 1.01 g of KNO3 (99%, Aldrich) were dissolved in ~10 ml of water to form a solution with a molar ratio of Mn/K=3.0. Next, 5 g of KIT-6 was dispersed in 200 ml of n-hexane. After stirring at room temperature for 3 h, 5 ml of the Mn/K solution was added slowly with stirring. The mixture was stirred overnight, filtered and dried at room temperature until a completely dried powder was obtained. The sample was heated slowly to 500 °C (1 °C min−1), calcined at that temperature for 5 h with a cover in a normal crucible unless is specified54 and the resulting material treated three times with a hot aqueous KOH solution (2.0 M), to remove the silica template, followed by washing with water and ethanol several times, and then drying at 60 °C.
Bimodal α-MnO2 (surface area 58 m2 g−1, K0.06MnO2) with micropore and a single mesopore size of 3.6 nm was prepared by using mesoporous silica SBA-15 as a hard template. The SBA-15 was prepared according to a previous report56.
Bulk α-MnO2 (surface area 8 m2 g−1, K0MnO2) was prepared by the reaction between 325 mesh Mn2O3 (99.0%, Aldrich) and 6.0 M H2SO4 solution at 80 °C for 24 h, resulting in the disproportionation of Mn2O3 into a soluble Mn2+ species and the desired α-MnO2 product46.
Treatment of KIT-6 with KNO3 was carried out as follows: 1.01 g of KNO3 was dissolved in ~15 ml of water to form a KNO3 solution. Five grams of mesoporous KIT-6 was dispersed in 200 ml of n-hexane. After stirring at room temperature for 3 h, 5 ml of KNO3 solution was added slowly with stirring. The mixture was stirred overnight, filtered and dried at room temperature until a completely dried powder was obtained. The sample was heated slowly to 300 or 500 °C (1 °C min−1), calcined at that temperature for 5 h and the resulting material was washed with water and ethanol several times, and then dried at 60 °C overnight.
The synthesis method for hierarchical porous LiMn2O4 was similar to that of tetramodal α-MnO2. The main difference was to use 1.01 g of LiNO3 instead of KNO3. After impregnation into KIT-6, calcination and silica etching, porous LiMn2O4 was obtained.
Characterization
TEM studies were carried out using a JEOL JEM-2011, employing a LaB6 filament as the electron source, and an accelerating voltage of 200 keV. TEM images were recorded by a Gatan charge-coupled device camera in a digital format. Wide-angle PXRD data were collected on a Stoe STADI/P powder diffractometer operating in transmission mode with Fe Kα1 source radiation (λ=1.936 Å). Low-angle PXRD data were collected using a Rigaku/MSC, D/max-rB with Cu Kα1 radiation (λ=1.541 Å) operating in reflection mode with a scintillation detector. N2 adsorption–desorption analysis was carried out using a Micromeritics ASAP 2020. The typical sample weight used was 100–200 mg. The outgas condition was set to 300 °C under vacuum for 2 h, and all adsorption–desorption measurements were carried out at liquid nitrogen temperature (−196 °C). The original DFT method for the slit pore geometry was used to extract the pore size distribution from the adsorption branch using the Micromeritics software39,40,41,42. A Horvath–Kawazoe method was used to extract the microporosity44.
Mn and K contents were determined by chemical analysis using a Philips PU9400X atomic adsorption spectrometer. The average oxidation state of framework manganese in α-MnO2 samples was determined by a redox titration method57.
Electrochemistry
First, the cathode was constructed by mixing the active material (α-MnO2), Kynar 2801 (a copolymer based on polyvinylidene fluoride), and Super S carbon (MMM) in the weight ratio 80:10:10. The mixture was cast onto Al foil (99.5%, thickness 0.050 mm, Advent Research Materials, Ltd) from acetone using a Doctor-Blade technique. After solvent evaporation at room temperature and heating at 80 °C under vacuum for 8 h, the cathode was assembled into cells along with a Li metal anode and electrolyte (Merck LP30, 1 M LiPF6 in 1:1 v/v ethylene carbonate/dimethyl carbonate). The cells were constructed and handled in an Ar-filled MBraun glovebox (O2<0.1 p.p.m., H2O<0.1 p.p.m.). Electrochemical measurements were carried out at 30 °C using a MACCOR Series 4200 cycler.
Catalysis
Catalytic CO oxidation was tested in a plug-flow microreactor (Altamira AMI 200). Fifty milligrams of catalyst was loaded into a U-shaped quartz tube (4 mm i.d.). After the catalyst was pretreated in flowing 8% O2 (balanced with He) at 400 °C for 1 h, the catalyst was then cooled down, the gas stream switched to 1% CO (balanced with air) and the reaction temperature ramped using a furnace (at a rate of 1 °C min−1 above ambient temperature) to record the light-off curve. The flow rate of the reactant stream was 37 cm3 min−1. A portion of the product stream was extracted periodically with an automatic sampling valve and was analysed using a dual column gas chromatograph with a thermal conductivity detector.
To perform N2O decomposition reaction testing, 0.5 g catalyst was packed into a U-shaped glass tube (7 mm i.d.) sealed by quartz wool, and pretreated in flowing 20% O2 (balance He) at 400 °C for 1 h (flow rate: 50 cm3 min−1). After cooling to near-room temperature, a gas stream of 0.5% N2O (balance He) flowed through the catalyst at a rate of 60 cm3 min−1, and the existing stream was analysed by a gas chromatograph (Agilent 7890A) that separates N2O, O2 and N2. The reaction temperature was varied using a furnace, and kept at 100, 150, 200, 250, 300, 350 and 400 °C for 30 min at each reaction temperature. The N2O conversion determined from GC analysis was denoted as X=([N2O]in—[N2O]out)/[N2O]in × 100%.
Additional Information
How to cite this article: Ren, Y. et al. A solid with a hierarchical tetramodal micro-meso-macro pore size distribution. Nat. Commun. 4:2015 doi: 10.1038/ncomms3015 (2013).
References
Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97, 2373–2419 (1997).
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
Taguchi, A. & Schüth, F. Ordered mesoporous materials in catalysis. Micro. Meso. Mater. 77, 1–45 (2005).
Férey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 37, 191–214 (2008).
Bruce, P. G. Scrosati, B. & Tarascon, J. M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008).
Zhai, Y. et al. Carbon materials for chemical capacitive energy storage. Adv. Mater. 23, 4828–4850 (2011).
Tüysüz, H. & Schüth, F. in Advances in Catalysis Chapter Two Vol. 55 pp 127–239 (Academic Press 2012).
Ren, Y. Ma, Z. & Bruce, P. G. Ordered mesoporous metal oxides: synthesis and applications. Chem. Soc. Rev. 41, 4909–4927 (2012).
Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 216, 298–312 (2003).
Liu, Y. Zhang, W. & Pinnavaia, T. J. Steam-stable aluminosilicate mesostructures assembled from zeolite type Y seeds. J. Am. Chem. Soc. 122, 8791–8792 (2000).
Meng, X. J. Nawaz, F. & Xiao, F. S. Templating route for synthesizing mesoporous zeolites with improved catalytic properties. Nano Today 4, 292–301 (2009).
Lopez-Orozco, S. Inayat, A. Schwab, A. Selvam, T. & Schwieger, W. Zeolitic materials with hierarchical porous structures. Adv. Mater. 23, 2602–2615 (2011).
Na, K. et al. Directing zeolite structures into hierarchically nanoporous architectures. Science 333, 328–332 (2011).
Chen, L. -H. et al. Hierarchically structured zeolites: synthesis, mass transport properties and applications. J. Mater. Chem. 22, 17381–17403 (2012).
Tsapatsis, M. Toward high-throughput zeolite membranes. Science 334, 767–768 (2011).
Zhang, X. et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 336, 1684–1687 (2012).
Jiang, J. et al. Synthesis and structure determination of the hierarchical meso-microporous zeolite ITQ-43. Science 333, 1131–1134 (2011).
Zhao, Y. et al. Metal–organic framework nanospheres with well-ordered mesopores synthesized in an ionic liquid/CO2/surfactant system. Angew. Chem. Int. Ed. 50, 636–639 (2011).
Feng, Q. Kanoh, H. & Ooi, K. Manganese oxide porous crystals. J. Mater. Chem. 9, 319–333 (1999).
Tiemann, M. Repeated templating. Chem. Mater. 20, 961–971 (2008).
Suib, S. L. Structure, porosity, and redox in porous manganese oxide octahedral layer and molecular sieve materials. J. Mater. Chem. 18, 1623–1631 (2008).
Zheng, H. et al. Nanostructured tungsten oxide – properties, synthesis, and applications. Adv. Funct. Mater. 21, 2175–2196 (2011).
Laha, S. C. & Ryoo, R. Synthesis of thermally stable mesoporous cerium oxide with nanocrystalline frameworks using mesoporous silica templates. Chem. Commun. 39, 2138–2139 (2003).
Tian, B. Z. et al. General synthesis of ordered crystallized metal oxide nanoarrays replicated by microwave-digested mesoporous silica. Adv. Mater. 15, 1370–1374 (2003).
Zhu, K. K. Yue, B. Zhou, W. Z. & He, H. Y. Preparation of three-dimensional chromium oxide porous single crystals templated by SBA-15. Chem. Commun. 39, 98–99 (2003).
Tian, B. Z. et al. Facile synthesis and characterization of novel mesoporous and mesorelief oxides with gyroidal structures. J. Am. Chem. Soc. 126, 865–875 (2004).
Jiao, F. Shaju, K. M. & Bruce, P. G. Synthesis of nanowire and mesoporous low-temperature LiCoO2 by a post-templating reaction. Angew. Chem. Int. Ed. 44, 6550–6553 (2005).
Rossinyol, E. et al. Nanostructured metal oxides synthesized by hard template method for gas sensing applications. Sens. Actuator B Chem. 109, 57–63 (2005).
Shen, W. H. Dong, X. P. Zhu, Y. F. Chen, H. R. & Shi, J. L. Mesoporous CeO2 and CuO-loaded mesoporous CeO2: Synthesis, characterization, and CO catalytic oxidation property. Micro. Meso. Mater. 85, 157–162 (2005).
Wang, Y. Q. et al. Weakly ferromagnetic ordered mesoporous Co3O4 synthesized by nanocasting from vinyl-functionalized cubic Ia3d mesoporous silica. Adv. Mater. 17, 53–56 (2005).
Ren, Y. et al. Ordered crystalline mesoporous oxides as catalysts for CO oxidation. Catal. Lett. 131, 146–154 (2009).
Jiao, K. et al. Growth of porous single-crystal Cr2O3 in a 3-D mesopore system. Chem. Commun. 41, 5618–5620 (2005).
Rumplecker, A. Kleitz, F. Salabas, E. L. & Schüth, F. Hard templating pathways for the synthesis of nanostructured porous Co3O4 . Chem. Mater. 19, 485–496 (2007).
Jiao, F. et al. Synthesis of ordered mesoporous NiO with crystalline walls and a bimodal pore size distribution. J. Am. Chem. Soc. 130, 5262–5266 (2008).
Ren, Y. Armstrong, A. R. Jiao, F. & Bruce, P. G. Influence of size on the rate of mesoporous electrodes for lithium batteries. J. Am. Chem. Soc. 132, 996–1004 (2010).
Ren, Y. Bruce, P. G. & Ma, Z. Solid-solid conversion of ordered crystalline mesoporous metal oxides under reducing atmosphere. J. Mater. Chem. 21, 9312–9318 (2011).
Ren, Y. Ma, Z. & Bruce, P. G. Ordered mesoporous NiMn2Ox with hematite or spinel structure: synthesis and application in electrochemical energy storage and catalytic conversion of N2O. Cryst. Eng. Comm. 13, 6955–6959 (2011).
Ren, Y. Ma, Z. & Bruce, P. G. Ordered mesoporous NiCoMnO4: Synthesis and application in energy storage and catalytic decomposition of N2O. J. Mater. Chem. 22, 15121–15127 (2012).
Balbuena, P. B. & Gubbins, K. E. Classification of adsorption behavior: simple fluids in pores of slit-shaped geometry. Fluid Phase Equilibria 76, 21–35 (1992).
Olivier, J. P. Modeling physical adsorption on porous and nonporous solids using density functional theory. J. Porous Mater. 2, 9–17 (1995).
Maddox, M. W. Olivier, J. P. & Gubbins, K. E. Characterization of MCM-41 using molecular simulation: Heterogeneity effects. Langmuir 13, 1737–1745 (1997).
Olivier, J. P. Improving the models used for calculating the size distribution of micropore volume of activated carbons from adsorption data. Carbon 36, 1469–1472 (1998).
Thommes, M. Cychosz, K. A. & Neimark, A. V. in Novel Carbon Adsorbents (ed Tascón, J. M. D.) pp107–145 (Elsevier: Oxford, 2012).
Horvath, G. & Kawazoe, K. Method for the calculation of effective pore-size distribution in molecular-sieve carbon. J. Chem. Eng. Jpn 16, 470–475 (1983).
Valdes-Solis, T. & Fuertes, A. B. High-surface area inorganic compounds prepared by nanocasting techniques. Mater. Res. Bull. 41, 2187–2197 (2006).
Johnson, C. S. et al. Structural and electrochemical studies of alpha-manganese dioxide (α-MnO2). J. Power Sources 68, 570–577 (1997).
Johns, P. A. Roberts, M. R. Wakizaka, Y. Sanders, J. H. & Owen, J. R. How the electrolyte limits fast discharge in nanostructured batteries and supercapacitors. Electrochem. Commun. 11, 2089–2092 (2009).
Ren, Y. Hardwick, L. J. & Bruce, P. G. Lithium intercalation into mesoporous anatase with an ordered 3d pore structure. Angew. Chem. Int. Ed. 49, 2570–2574 (2010).
Gac, W. Giecko, G. Pasieuna-Patkowska, S. Borowiecki, T. & Kepinski, L. The influence of silver on the properties of cryptomelane type manganese oxides in N2O decomposition reaction. Catal. Today 137, 397–402 (2008).
Jiao, F. Bao, J. L. Hill, A. H. & Bruce, P. G. Synthesis of ordered mesoporous Li-Mn-O spinel as a positive electrode for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 9711–9716 (2008).
Kleitz, F. Choi, S. H. & Ryoo, R. Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 39, 2136–2137 (2003).
Sakamoto, Y. Kim, T. W. Ryoo, R. & Terasaki, O. Three-dimensional structure of large-pore mesoporous cubic Ia3d silica with complementary pores and its carbon replica by electron crystallography. Angew. Chem. Int. Ed. 43, 5231–5234 (2004).
Kim, T. W. Kleitz, F. Paul, B. & Ryoo, R. MCM-48-like large mesoporous silicas with tailored pore structure: facile synthesis domain in a ternary triblock copolymer-butanol-water system. J. Am. Chem. Soc. 127, 7601–7610 (2005).
Sun, X. et al. Container effect in nanocasting synthesis of mesoporous metal oxides. J. Am. Chem. Soc. 133, 14542–14545 (2012).
Ren, Y. Jiao, F. & Bruce, P. G. Tailoring the pore size/wall thickness of mesoporous transition metal oxides. Micro. Meso. Mater. 121, 90–94 (2009).
Zhao, D. Y. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998).
Vogel, A. I. Vogel’s Textbook of Quantitative Inorganic Analysis, Including Elementary Instrumental Analysis 3rd edn Longman: London, (1978).
Acknowledgements
Y.R. is indebted to the Russell Trust for a travel grant. Z.M. is grateful for the financial support by the National Natural Science Foundation of China (grant numbers 21007011 and 21177028) and the PhD programmes Foundation of the Ministry of Education in China (grant number 20100071120012). F.J. would like to acknowledge the startup fund from the University of Delaware. S.D. is sponsored by the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences, US Department of Energy. P.G.B. is grateful to the EPSRC, including SUPERGEN and the programme grant ‘Nanoionics’ for financial support.
Author information
Authors and Affiliations
Contributions
Y.R. conducted all of the synthesis and most of the characterization. Z.M. and S.D. carried out the catalytic studies. Z.L. and F.J. assisted with gas adsorption. Y.R., R.E.M., Z.M. and P.G.B. interpreted the results and wrote the manuscript. Y.R. and P.G.B. initiated and guided the work. All authors contributed to the discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1-S8 and Supplementary Table S1 (PDF 958 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ren, Y., Ma, Z., Morris, R. et al. A solid with a hierarchical tetramodal micro-meso-macro pore size distribution. Nat Commun 4, 2015 (2013). https://doi.org/10.1038/ncomms3015
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms3015
This article is cited by
-
Applying Circular Economy to Construction Industry through Use of Waste Materials: A Review of Supplementary Cementitious Materials, Plastics, and Ceramics
Circular Economy and Sustainability (2022)
-
Performance and kinetic study on Pd/OMS-2 catalyst for CO catalytic oxidation: effect of preparation method
Research on Chemical Intermediates (2017)
-
Effects of precipitants and surfactants on catalytic activity of Pd/CeO2 for CO oxidation
Research on Chemical Intermediates (2017)
-
Temperature-mediated phase transformation, pore geometry and pore hysteresis transformation of borohydride derived in-born porous zirconium hydroxide nanopowders
Scientific Reports (2016)
-
Mesoporous materials for energy conversion and storage devices
Nature Reviews Materials (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.