Abstract
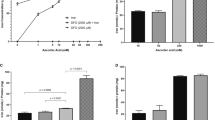
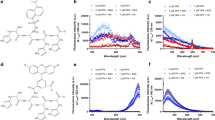
Improved methods for studying intracellular reactive Fe(II) are of significant interest for studies of iron metabolism and disease-relevant changes in iron homeostasis. Here we describe a highly selective reactivity-based probe in which a Fenton-type reaction with intracellular labile Fe(II) leads to unmasking of the aminonucleoside puromycin. Puromycin leaves a permanent and dose-dependent mark on treated cells that can be detected with high sensitivity and precision using a high-content, plate-based immunofluorescence assay. Using this new probe and screening approach, we detected alteration of cellular labile Fe(II) in response extracellular iron conditioning, overexpression of iron storage and/or export proteins, and post-translational regulation of iron export. We also used this new tool to demonstrate that labile Fe(II) pools are larger in cancer cells than in nontumorigenic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Torti, S.V. & Torti, F.M. Iron and cancer: more ore to be mined. Nat. Rev. Cancer 13, 342–355 (2013).
Bandyopadhyay, S., Chandramouli, K.K. & Johnson, M.K. Iron-sulphur cluster biosynthesis. Biochem. Soc. Trans. 36, 1112–1119 (2008).
Johnson, D.C., Dean, D.R., Smith, A.D. & Johnson, M.K. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 74, 247–281 (2005).
Kurz, T., Eaton, J.W. & Brunk, U.T. The role of lysosomes in iron metabolism and recycling. Int. J. Biochem. Cell Biol. 43, 1686–1697 (2011).
O'Neill, P.M. & Posner, G.H. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 47, 2945–2964 (2004).
Mercer, A.E. et al. Evidence for the involvement of carbon-centered radicals in the induction of apoptotic cell death by artemisinin compounds. J. Biol. Chem. 282, 9372–9382 (2007).
Dixon, S.J. & Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014).
Pantopoulos, K. Iron metabolism and the IRE/IRP regulatory system: an update. Ann. NY Acad. Sci. 1012, 1–13 (2004).
Wang, J. & Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 434, 365–381 (2011).
Richardson, D.R. & Ponka, P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 1331, 1–40 (1997).
Fernaeus, S. & Land, T. Increased iron-induced oxidative stress and toxicity in scrapie-infected neuroblastoma cells. Neurosci. Lett. 382, 217–220 (2005).
Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 30, 105–122 (2010).
Boult, J. et al. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin. Cancer Res. 14, 379–387 (2008).
Kakhlon, O., Gruenbaum, Y. & Cabantchik, Z.I. Ferritin expression modulates cell cycle dynamics and cell responsiveness to H-ras-induced growth via expansion of the labile iron pool. Biochem. J. 363, 431–436 (2002).
Pinnix, Z.K. et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2, 43ra56 (2010).
Wu, K.J., Polack, A. & Dalla-Favera, R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science 283, 676–679 (1999).
Toyokuni, S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 100, 9–16 (2009).
Miller, L.D. et al. An iron regulatory gene signature predicts outcome in breast cancer. Cancer Res. 71, 6728–6737 (2011).
Kakhlon, O. & Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 33, 1037–1046 (2002).
Epsztejn, S. et al. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 248, 31–40 (1997).
Petrat, F., de Groot, H., Sustmann, R. & Rauen, U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 383, 489–502 (2002).
Hirayama, T., Okuda, K. & Nagasawa, H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 4, 1250–1256 (2013).
Au-Yeung, H.Y., Chan, J., Chantarojsiri, T. & Chang, C.J. Molecular imaging of labile iron(II) pools in living cells with a turn-on fluorescent probe. J. Am. Chem. Soc. 135, 15165–15173 (2013).
Aron, A.T., Ramos-Torres, K.M., Cotruvo, J.A. & Chang, C.J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 48, 2434–2442 (2015).
Valecha, N. et al. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: a phase II, multicenter, randomized, dose-finding clinical trial. Clin. Infect. Dis. 51, 684–691 (2010).
Borstnik, K., Paik, I., Shapiro, T.A. & Posner, G.H. Antimalarial chemotherapeutic peroxides: artemisinin, yingzhaosu A and related compounds. Int. J. Parasitol. 32, 1661–1667 (2002).
Valecha, N. et al. Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated plasmodium falciparum malaria: a comparative, multicenter, randomized clinical trial. Clin. Infect. Dis. 55, 663–671 (2012).
Charman, S.A. et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci. USA 108, 4400–4405 (2011).
Creek, D.J. et al. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob. Agents Chemother. 52, 1291–1296 (2008).
Creek, D.J. et al. Iron-mediated degradation kinetics of substituted dispiro-1, 2, 4-trioxolane antimalarials. J. Pharm. Sci. 96, 2945–2956 (2007).
Tang, Y. et al. Dispiro-1,2,4-trioxane analogues of a prototype dispiro-1,2,4-trioxolane: mechanistic comparators for artemisinin in the context of reaction pathways with iron(II). J. Org. Chem. 70, 5103–5110 (2005).
Wang, X. et al. Spiroadamantyl 1,2,4-trioxolane, 1,2,4-trioxane, and 1,2,4-trioxepane pairs: relationship between peroxide bond iron(II) reactivity, heme alkylation efficiency, and antimalarial activity. Bioorg. Med. Chem. Lett. 19, 4542–4545 (2009).
Mahajan, S.S. et al. A fragmenting hybrid approach for targeted delivery of multiple therapeutic agents to the malaria parasite. ChemMedChem 6, 415–419 (2011).
Fontaine, S.D., Dipasquale, A.G. & Renslo, A.R. Efficient and stereocontrolled synthesis of 1,2,4-trioxolanes useful for ferrous iron-dependent drug delivery. Org. Lett. 16, 5776–5779 (2014).
Fontaine, S.D. et al. Drug delivery to the malaria parasite using an arterolane-like scaffold. ChemMedChem 10, 47–51 (2015).
Petrat, F., Rauen, U. & de Groot, H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology 29, 1171–1179 (1999).
Chen, C. & Paw, B.H. Cellular and mitochondrial iron homeostasis in vertebrates. Biochim. Biophys. Acta 1823, 1459–1467 (2012).
Yan, C.Y., Ferrari, G. & Greene, L.A. N-acetylcysteine-promoted survival of PC12 cells is glutathione-independent but transcription-dependent. J. Biol. Chem. 270, 26827–26832 (1995).
Mukherjee, T.K., Mishra, A.K., Mukhopadhyay, S. & Hoidal, J.R. High concentration of antioxidants N-acetylcysteine and mitoquinone-Q induces intercellular adhesion molecule 1 and oxidative stress by increasing intracellular glutathione. J. Immunol. 178, 1835–1844 (2007).
Frikke-Schmidt, H. & Lykkesfeldt, J. Keeping the intracellular vitamin C at a physiologically relevant level in endothelial cell culture. Anal. Biochem. 397, 135–137 (2010).
Gao, J.P., Friedman, S. & Lanks, K.W. The role of reduced nicotinamide adenine dinucleotide phosphate in glucose dependent and temperature dependent doxorubicin cytotoxicity. Cancer Chemother. Pharmacol. 33, 191–196 (1993).
Ishii, T., Sugita, Y. & Bannai, S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J. Cell. Physiol. 133, 330–336 (1987).
Kang, Y.J., Feng, Y.I. & Hatcher, E.L. Glutathione stimulates A549 cell proliferation in glutamine-deficient culture: the effect of glutamate supplementation. J. Cell. Physiol. 161, 589–596 (1994).
Espósito, B.P., Epsztejn, S., Breuer, W. & Cabantchik, Z.I. A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal. Biochem. 304, 1–18 (2002).
Martins, M.M. et al. Linking tumor mutations to drug responses via a quantitative chemical-genetic interaction map. Cancer Discov. 5, 154–167 (2015).
Acknowledgements
The authors thank S. Chen for technical support in automated cell imaging and image analysis with IN Cell Developer software, T. Matsuguchi for assistance with qRT-PCR setup and analysis, H. Shimizu for assistance with the PAMPA assay, and D. Ruggero for helpful comments on the manuscript. A.R.R. is funded by US National Institutes of Health (NIH) grant AI105106. C.J.C. is funded by NIH grant GM 79465. B.S. and C.W.M. acknowledge funding from the NIH Research Training Grant in Chemistry and Chemical Biology (T32 GM064337).
Author information
Authors and Affiliations
Contributions
B.S., J.A.W., and A.R.R. conceived and designed experiments. B.S., C.W.M. and S.D.F. carried out experiments. B.S., C.W.M., S.D.F., J.A.W., and A.R.R. analyzed data. B.S., C.W.M., and A.R.R. wrote the manuscript. M.N.V.W. and C.J.C. provided reagents and discussion. All authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.S., S.D.F., J.A.W., and A.R.R. are listed as inventors on a patent application describing 3 and related trioxolane conjugates.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–9 and Supplementary Table 1. (PDF 2897 kb)
Supplementary Note
Synthetic Procedures (PDF 549 kb)
Rights and permissions
About this article
Cite this article
Spangler, B., Morgan, C., Fontaine, S. et al. A reactivity-based probe of the intracellular labile ferrous iron pool. Nat Chem Biol 12, 680–685 (2016). https://doi.org/10.1038/nchembio.2116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2116
This article is cited by
-
CYLD regulates cell ferroptosis through Hippo/YAP signaling in prostate cancer progression
Cell Death & Disease (2024)
-
Spectroscopic Investigation of Coumarin Based Novel Fluorescent TURN OFF Sensor for the Selective Detection of Fe3+: In-vitro Live Cell Imaging Application
Journal of Fluorescence (2024)
-
The mechanism of ferroptosis and its related diseases
Molecular Biomedicine (2023)
-
Rhodamine-based Fluorescent Probe With Quick Response and High Selectivity for Imaging Labile Ferrous Iron in Living Cells and Zebrafish
Journal of Fluorescence (2023)
-
A puromycin-dependent activity-based sensing probe for histochemical staining of hydrogen peroxide in cells and animal tissues
Nature Protocols (2022)