Abstract
Labile copper(II) ions (Cu2+) in serum are considered to be readily available for cellular uptake and to constitute the biologically active Cu2+ species in the blood. It might also be suitable to reflect copper dyshomeostasis during diseases such as Wilson’s disease (WD) or neurological disorders. So far, no direct quantification method has been described to determine this small Cu2+ subset. This study introduces a fluorometric high throughput assay using the novel Cu2+ binding fluoresceine-peptide sensor FP4 (Kd of the Cu2+-FP4-complex 0.38 pM) to determine labile Cu2+ in human and rat serum. Using 96 human serum samples, labile Cu2+was measured to be 0.14 ± 0.05 pM, showing no correlation with age or other serum trace elements. No sex-specific differences in labile Cu2+ concentrations were noted, in contrast to the total copper levels in serum. Analysis of the effect of drug therapy on labile Cu2+ in the sera of 19 patients with WD showed a significant decrease in labile Cu2+ following copper chelation therapy, suggesting that labile Cu2+ may be a specific marker of disease status and that the assay could be suitable for monitoring treatment progress.
Similar content being viewed by others
Introduction
The essential trace element copper is indispensable for various physiological functions, such as support of oxidative phosphorylation, antioxidant activity, formation of several hormones, and iron metabolism1,2. The metal is distributed throughout the body via the bloodstream, and 0.75–1.4 mg/L Cu2+ can be found in human serum3,4. Approximately 70–90% thereof is contained in ceruloplasmin (CP), while the remaining Cu2+, which is typically being referred to as loosely bound Cu2+, is associated with albumin (10–15%), α-macroglobulin (5–15%), clotting factors, enzymes (superoxide dismutase (SOD), Oxidases), metallothionein, as well as small Cu2+ carriers3,4,5. The loosely bound Cu2+ species comprises the whole amount of serum Cu2+ that is not bound to CP, whereas so-called labile Cu2+ represents a smaller subset of the loosely bound pool and is defined only to be in equilibrium with low molecular weight (LMW) ligands, e.g., amino acids. This labile Cu2+ pool is considered to be readily available for cellular uptake and was even discussed to cross the blood–brain barrier as LMW-Cu2+-complexes6. As copper is redox active, an increase of labile Cu2+ must be tightly controlled to prevent formation of reactive oxygen species and tissue damage1,7.
Diseases associated with copper dishomeostasis and changes in serum Cu2+ are Menkes, Wilson’s (WD)8,9, cancer10, and neurodegenerative diseases1,11,12,13,14,15,16, such as Parkinson’s and Alzheimer’s disease. Particularly in the copper storage disorder WD8,9 and neurodegenerative diseases1,11,12,13,15 both loosely bound and labile levels of serum Cu2+ were found to be elevated. Hence, these serum Cu2+ species are considered to serve as promising diagnostic markers for disease-related alterations of copper homeostasis6,13.
In addition to indirect quantification of loosely bound or non-CP-bound Cu2+ in serum by determination of CP content and total Cu2+ content15,17,18, several experimental approaches for direct measurement of this Cu2+ species have been developed to date. This Cu2+ pool, which is also often defined as extractable or exchangeable Cu2+, has been either directly quantified by liquid chromatography (LC) coupled to inductively coupled plasma-mass spectrometry (ICP-MS)19,20, or after extracting the loosely bound metal in serum with Cu2+ chelators such as EDTA9,21 or Cu2+-affine resins22,23 followed by size ultrafiltration or size exclusion chromatography (SEC) and quantified either by ICP-MS21,22,23, atomic absorption spectrometry (AAS)9,23, or fluorescent Cu2+ sensors13. The resulting concentration of loosely bound Cu2+ was in the range of 0.5–7 µM9,13,19,20,21,22,23. Up to now, the smaller serum Cu2+ fraction that is not bound to CP or albumin, but in equilibrium with the remainder of Cu2+ binding compounds in serum, was quantified by means of ultrafiltration followed by direct measurement of copper by AAS6,24,25 or ICP-MS8. This fraction is in the nanomolar concentration range6 and has often been equated with labile Cu2+. However, there is at present no suitable method allowing direct quantification of labile Cu2+ in serum without a prior extraction step. The use of metal-responsive fluorescent sensors to quantify free metal species in biofluids represents a suitable approach to directly measure metal cations in serum while requiring small sample volumes26. Therefore, the aim of this study was to establish a fluorescence-based method for determining the concentration of labile Cu2+ in serum samples with a small sample volume and a high throughput.
Materials and methods
Materials
Chelex® 100 resin (Bio-Rad, Hercules, USA), CuSO4, Dimethylsulfoxide (DMSO), Ethylenediaminetetraacetic acid (EDTA), Ethylene glycol bis(2-aminoethyl ether)tetraacetic acid (EGTA), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), histidine, were purchased from Sigma Aldrich (Munich, Germany). All other materials were from standard sources and of analytical purity.
Fluorescent sensors
Fluorescein peptide 4 (FP4) was synthesized by Peptide Specialty Laboratories GmbH (Heidelberg, Germany). Dansyl peptide 4 (DP4) was synthesized by manual solid phase peptide synthesis (SPPS) using a standard Fmoc-strategy. Fmoc-K(DNS)-OH was used as starting material for the SPPS and synthesized according to the method of Williamson et al.27 (for details refer to Supplementary Sect. 1). Stock solutions of FP4 and DP4 (1 mM, in DMSO) were aliquoted and stored at −20 °C. Each aliquot was thawed only twice.
Determination of Cu2+ binding affinity
Determination of the dissociation constant of the Cu2+-FP4-complex was done as described28,29 using histidine and EGTA as competitors for Cu2+ binding. Experiments were performed in assay buffer, consisting of 50 mM HEPES, pH 7.4, depleted from bivalent metal ions by treatment with Chelex® 100 resin26. To determine the aqueous Cu2+ concentration, CHEAQS Next 2014-2020 software and the NIST Database 46 Version 8.0 was applied, using log KA for Cu2+-histidine, Cu2+-histidine2, and Cu2+-EGTA at pH 7.4 from Young et al.28 and log KA for Cu2+-HEPES from Sokołowska et al.30 (Supplementary Table 1).
Human serum samples
A commercially available standard serum derived from a mixture of human serum samples was used as reference serum (in.vent Diagnostica GmbH, Hennigsdorf, Germany). A set of commercially available individual human serum samples (N = 96, Table 1) (in.vent Diagnostica GmbH, Hennigsdorf, Germany) served as a reference cohort for healthy individuals within this study.
Serum samples of WD patients were obtained from 19 patients (Table 1) at the time point of disease diagnosis and from the same patients after initiation of medical treatment. Mean treatment duration till second time point under therapy was 72.9 (range 6–144) months. Patients were recruited between 2010 and 2018 at the University Hospital Heidelberg, Germany, as part of the clinical trial ‘Biochemical and genetic markers of liver diseases’. Clinical parameters of the investigated human WD patients and the respective medical treatment are listed in Supplementary Tables 2 and 3. The study was approved by the ethics committee of the University of Heidelberg and informed consent to participate in the study was obtained from each subject. The study was carried out in accordance with the Declaration of Helsinki.
Rat serum samples
Control Atp7b+/− LPP rats (N = 5; crossbreed between Long Evans cinnamon rats and Piebald Virol Glaxo rats) were fed ad libitum with standard rat chow (Altromin Spezialfutter GmbH, Seelenkamp, Germany) and tap water31. At the age of 81–93 days, animals were sacrificed, and serum was collected. Experiments were approved by the government authorities of the Regierung von Oberbayern, Munich, Germany. Animals were maintained under the Guidelines for the Care and Use of Laboratory Animals of the Helmholtz Center Munich. All methods are reported in accordance with ARRIVE guidelines.
Labile serum Cu2+
Similar to the labile zinc (Zn2+) assay reported by Alker et al.26, 50 mM HEPES buffer, pH 7.4, bivalent metal ion-depleted with Chelex® 100 resin, was used in all steps. 20 µL human or rat serum, pre-diluted to 5% in ice-cold assay buffer, were added to 10 nM FP4 in 80 µL assay buffer in black 96-well plates (Brand, Wertheim, Germany) and gently shaken in the dark. During the assay, only the inner 60 wells were used, and each sample was analyzed in triplicates. The outer wells were filled with distilled water to ensure a uniform temperature over the entire plate. After 60 min, the fluorescence signal (F) of FP4 was measured using a SPARK Tecan plate reader (Tecan, Switzerland) at λex = 495 nm and λEm = 523 nm. Subsequently, 5 µL of 42 mM EDTA (diluted in assay buffer) was added to the wells, resulting in a final concentration of 2 mM EDTA, and incubated for additional 60 min. After measuring the maximum fluorescence signal of the sensor without any bound Cu2+ (Fapo), the minimum fluorescence signal (FCu) was generated by adding 5 µL of 48.4 mM CuSO4 (in distilled water), corresponding to a final Cu2+ concentration of 2.2 mM, and the fluorescence intensity was determined after incubation for further 60 min. All steps were performed in the dark and at room temperature (25 °C). The labile Cu2+ concentration was calculated according to Grynkiewicz et al.32 by multiplying [(Fapo − F)/(F − FCu)] with the dissociation constant of the Cu2+-FP4-complex of 0.38 pM, determined in this study.
Total trace element levels in serum
Concentrations of total selenium, copper, and zinc in the serum samples were quantified with total reflection X-ray fluorescence (TXRF) using a benchtop TXRF spectrometer (S4 T-STAR, Bruker Nano GmbH, Berlin, Germany) as previously described33,34.
Labile serum Zn2+
The concentration of labile Zn2+ was determined by a fluorometric method using the low molecular weight Zn2+ sensor Zinpyr-1 (Santa Cruz biotechnology, Dallas, USA) as described26.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 9.3.1 (GraphPad Software Inc., San Diego, CA, USA). Data were tested for normal distribution using the Shapiro–Wilk test. Correlations were analyzed using Spearman correlation analysis. Statistically significant differences between two means were identified with t-test for parametric or Mann–Whitney test for non-parametric data, or between three or more means by one-way analysis of variance (ANOVA) followed by Tukey's multiple post hoc comparison test or non-parametric Kruskal–Wallis with Dunn’s multiple comparison test. Differences were considered significant if p values were *p < 0.05, **p < 0.01, or ***p < 0.001, as indicated in the figure legends. Error bars represent standard deviation (SD) of at least three independent experiments.
Results and discussion
Choice of Cu2+-responsive fluorescent sensor
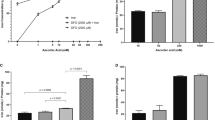
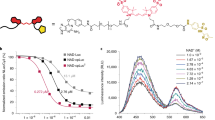
A metal-responsive sensor for the detection and quantification of labile Cu2+ in serum must exhibit Cu2+-dependent fluorescence changes, high Cu2+-selectivity and -sensitivity, but also have suitable Cu2+ affinity and reversible binding of the metal35. In addition, good water solubility and negligible interaction with the complex biomatrix are required for the application of such sensors in biofluids, such as serum containing proteins, lipids, and carbohydrates. A suitable fluorescence yield (Ф) and extinction coefficient (ε), which defines the brightness of the fluorophore = Ф* ε35,36 is another crucial requirement for the physicochemical properties of sensors. Initially, the peptide-based dansyl Cu2+ sensors developed by Young et al. were chosen as they seemed to meet all prerequisites28. Unfortunately, DP4 (Fig. 1a) turned out to be poorly suited for detecting labile Cu2+ in serum samples, as the absorption of serum proteins interfered with fluorescence of the dansyl sensor. According to the excitation and emission spectra of DP4 in the presence of 1% HS or correspondingly diluted physiological serum albumin levels, added as 0.5 g/L bovine serum albumin (BSA), this was mainly due to the albumin content of serum (Fig. 1b, c). To circumvent this interference, the dansyl fluorophore of the peptide sensor was replaced with carboxyfluorescein (FAM), which emits light of lower energy and has a higher fluorescence yield and extinction coefficient than the dansyl molecule28,37, leading to the sensor FP4 (Fig. 1d). In contrast to DP4, FP4 was undisturbed by any autofluorescence or absorbance of serum proteins. Comparison of FP4 in the presence and absence of HS shows that the emission and excitation spectra were not affected by the presence of serum (Fig. 1e, f). Furthermore, the metal selectivity of the probe was assessed (Supplementary Fig. 1). No physiologically relevant cation in serum had an effect on sensor fluorescence or Cu2+ binding by FP4. However, FP4 fluorescence was quenched by adding a 20-fold excess of Ni2+ to FP4, yet the applied concentrations do not represent physiological nickel levels in serum38. Furthermore, subsequent addition of Cu2+ resulted in a decrease in fluorescence comparable to that observed with FP4 and Cu2+ alone.
Dissociation constant of the Cu2+-FP4-complex
To assess whether introduction of FAM did influence the Cu2+-affinity of the probe, the dissociation constant of FP4 was determined with EGTA and histidine by a similar experimental approach as the one applied by Young et al.28, yielding a conditional log(Kd) = −12.416 for the Cu2+-FP4-complex, corresponding to 0.38 pM (Fig. 2, Supplementary Table 1). Accordingly, the Cu2+-affinity of FP4 is lower than that of DP4, but in the vicinity of the hitherto reported labile Cu2+ levels in serum6,22 and thus suitable for determining this Cu2+ species.
Cu2+ binding affinity of FP4. Relative fluorescence of FP4 in the presence of different concentrations of EGTA (a) and histidine (c). Sigmoidal dose response of [Cu-FP4]/[FP4]tot and the labile Cu2+ concentration log [Cuaq2+] upon titration with chelators EGTA (b) or histidine (d). Shown are means ± standard deviation of three independent experiments.
Assay parameters
To minimize the perturbation of the equilibria between labile and bound Cu2+ in serum by the addition of another Cu2+ binding species added in form of the sensor, the probe concentration needs to be as low as possible26. To identify suitable concentrations of FP4, 0–100 nM FP4 were titrated to 1% human serum and baseline fluorescence (F) was measured, followed by detection of sensor fluorescence upon sequential addition of 2 mM EDTA as Cu2+ chelator and 2.2 mM Cu2+ to saturate the probe, generating Fapo and Fcu, respectively (Fig. 3). 10 nM FP4 were sufficient to induce a stable fluorescence signal distinguishable from the autofluorescence of serum and buffer (Fig. 3a) while providing maximum Fapo to F ratio (Fig. 3b). By determining the fractional saturation of the sensor in the presence of human serum and using the Kd for the Cu2+-FP4-complex of 0.38 pM, the labile Cu2+ level in the reference serum was 0.14 ± 0.02 pM when applying 10 nM sensor (Fig. 3c). The addition of 5–50 nM sensor had no effect on the calculated labile Cu2+ concentration, while the addition of excessive amounts of sensor (100 nM) considerably decreased the determined labile Cu2+ values (Fig. 3c). This confirms the importance of an optimized sensor concentration and is consistent with other studies on the influence of excessive sensor levels on the determined labile metal concentrations39,40.
Optimization of assay parameters. Assay parameters were tested in the presence of 1% human reference serum. (a) Fluorescence intensity depending on sensor concentration (F), after the addition of 2 mM EDTA (Fapo) and 2.2 mM CuSO4 (Fcu). (b) Fapo/F and Fcu/F ratios of 1–100 nM FP4 in the presence of 1%HS. (c) Labile Cu2+ (pM) in HS depending on sensor concentration. (d) Fluorescence of apo-FP4 in the presence of 1% HS as ratios of the maximal fluorescence upon addition of 0.5–2 mM EDTA or EGTA relative to the FP4 fluorescence at 60 min. (e) Time course of the fluorescence signal of FP4 in 1% HS for parameters F, Fapo (after addition of 2 mM EDTA), and Fcu (after addition of 2.1 mM or 2.2 mM CuSO4) relative to the fluorescence at t = 60 min. (f) Fluorescence of final parameters. Significant differences are indicated by *p < 0.05; *p < 0.01; ***p < 0.001 (a) (two way ANOVA with Sidak’s multiple comparisons test) or by letters (d,f), whereas bars sharing a letter are not significantly different (one way ANOVA with Tukey’s multiple comparisons test). Results are shown as means ± SEM/SD of at least three independent experiments.
After a suitable sensor concentration was found, the assay parameters F, Fapo, and Fcu had to be optimized with regard to incubation time and concentrations of Cu2+ and Cu2+ chelator, respectively. Comparison of the Cu2+ chelators EGTA and EDTA to measure the maximum fluorescence signal of the Cu2+-free sensor (Fapo) shows that 2 mM EDTA induced significantly higher fluorescence than lower EDTA concentrations, while no significant differences between the chelators and tested EGTA concentrations were observed. Accordingly, a final EDTA concentration of 2 mM was chosen to generate the Fapo signal in the final assay (Fig. 3d). After inducing Fapo, the addition of CuSO4 in excess (concentration per well: 2.2 mM) was required to fully saturate FP4 with Cu2+ and quench its fluorescence to yield the minimum fluorescence of the sensor (Fcu) (Fig. 3e). In order to optimize the incubation time required to generate the assay parameters F, Fapo, and Fcu, time-resolved measurements were carried out, showing that an incubation of 60 min each were sufficient to allow establishing an equilibrium for Cu2+ in the distribution between the ligands in serum and FP4, generating stable fluorescence signals for all three parameters in human (Fig. 3e) and rat serum (Supplementary Fig. 2).
According to the final assay protocol, the assay time is about 3 h for up to 19 samples per plate and with parallel and slightly staggered preparation of 4 plates, a total of 76 samples can be analyzed within 4 h. Each serum is tested in triplicate, which, including calculated dead volume, means a total sample volume requirement of only 5 µL serum. A human reference serum is carried on each plate as quality control. The intra- and inter-day reproducibility of the assay was investigated by measuring the human reference serum, with a labile Cu2+ level of 0.05 pM, and evaluated with a relative standard deviation of 16.3% (intra-day) and 21.6% (inter-day) of the determined labile Cu2+ levels, respectively (Fig. 4a). To also characterize the requirements of the assay with regard to sample quality, the influences of freeze-thawing cycles, storage temperature, and Cu2+ spiking of the reference serum on the final labile Cu2+ concentration were determined (Fig. 4b–d). Accordingly, storage of samples at −80 °C or −20 °C is required (Fig. 4b) while only a minimum number of freeze–thaw cycles are acceptable (Fig. 4c) to avoid affecting the labile Cu2+ content in serum. In addition, the test can also be used to determine the labile Cu2+ content in serum from other species, such as rat serum, where a labile Cu2+ level of 0.16 ± 0.03 pM was measured.
Stability of labile Cu2+ in serum. Repeatability and reproducibility of the assay are shown, including relative standard deviation (RSD) (a). Labile Cu2+ concentration in the reference serum depending on the number of freeze–thaw cycles (b) and storage temperature (c) are depicted. The labile Cu2+ concentration in 1% human reference serum upon spiking with 0 or 20 nM CuSO4 (N = 4) is presented (d). Statistically significant differences between labile Cu2+ values were determined with non-parametric Kruskal–Wallis with Dunn’s multiple comparison test (b), ordinary one way ANOVA followed by Tukey multiple comparison test (c), and unpaired t-test (*p < 0.05, **p < 0.01; ***p < 0.001). Results are presented as data points including mean ± SD of at least three independent experiments.
Application of the labile Cu2+ assay in a human cohort
In a cohort of 96 healthy human subjects, a mean labile serum Cu2+ level of 0.14 ± 0.05 pM, ranging from 0.05 to 0.37 pM, was determined by the FP4-based assay (Fig. 5a). Previous studies based on a two-step method involving the removal of Cu2+ bound to CP and albumin by ultracentrifugation followed by instrumental quantification of Cu2+ in the eluate, reported Cu2+ concentrations in the nanomolar range6,22. However, this approach does not exclude the Cu2+ subset bound to other serum components, such as metallothionein or enzymes, and is therefore not comparable to the labile Cu2+ species determined by our direct fluorometric assay. Moreover, labile Cu2+ and total serum Cu2+ did not correlate (Fig. 5a), suggesting that labile Cu2+ is not simply a subset of the total copper pool, but reflects a discrete Cu2+ pool that is affected by other serum components. Another indication that labile free Cu2+ species is a separate pool from the total Cu2+ is the fact that it was possible to detect changes in free Cu2+ against the background of the CP-bound Cu2+ pool. Already the addition of a relatively small amount of Cu2+ to the human reference serum had a significant effect on the concentration of available labile Cu2+, as spiking the reference serum (basal labile Cu2+ concentration of 0.05 pM) with only 20 nM Cu2+ resulted in a tripling of the labile Cu2+ level to 0.15 ± 0.02 pM (Fig. 4d). Still, the vast majority of the added Cu2+ was not detected in the labile fraction, confirming an interaction with ligands able to buffer these ions to a significant extent. This shows that the labile Cu2+ determined in serum by our method not only depends on the amount of loosely bound (i.e., non-CP-bound) Cu2+, but as well on the binding capacity of ligands with intermediate affinity.
Labile Cu2+ in sera from a cohort of healthy human subjects. Labile Cu2+ shown in relation to total copper (a) in serum of a ‘healthy’ human cohort (N = 96). Sex differences in total copper of the serum are shown (b). Labile Cu2+ concentrations in the human cohort (N = 96) are depicted according to sex (c) and age of donors (d), total selenium (e) and iron levels (f) as well as to labile Zn2+ (g) and total zinc (h). Additionally, correlation of total zinc and total copper serum levels is shown (i). Data are presented as means + SD, and p values are indicated [non-parametric test using Mann–Whitney test (b,c)], and as scatter plots, including Spearman correlation coefficients (r) and p-values.
In contrast to the significantly higher total serum Cu2+ concentration in sera of adult women than men (Fig. 5b), which has been well described in the literature41,42, labile Cu2+ levels did not differ significantly between female and male donors (Fig. 5c). Moreover, the labile Cu2+ species did not correlate with the age of donors (Fig. 5d). Correlation analysis of labile Cu2+ with total serum levels of selenium, iron or zinc did also not show any significant trend (Fig. 5e–g). In contrast, labile Zn2+ concentrations showed a weak negative correlation with labile Cu2+ levels in sera (Fig. 5h, r = −0.26, p = 0.02), which was not observed for total zinc and total copper levels in the same cohort (Fig. 5i).
Labile Cu2+ in Wilson’s disease
Finally, to assess the effect of a disease with copper dyshomeostasis on serum labile Cu2+, the labile Cu2+ assay was applied to sera from 19 WD patients before and after initiation of medical therapy. As medications zinc, D-penicillamine, or trientine were used, which are commonly applied treatments for WD and supposed to reduce and revert the harmful copper accumulation during the disease43. Clinical parameters of the investigated human WD patients and the respective medical treatment are listed in Supplementary Tables 2 and 3. The average labile Cu2+ level in serum of WD patients of 0.16 ± 0.08 pM was significantly reduced to 0.10 ± 0.07 pM labile Cu2+ under ongoing treatment (Fig. 6a). This is consistent with another study reporting decreased loosely bound copper levels upon treatment of WD8. Total serum Cu2+ only slightly decreased after medical treatment (Fig. 6b). Similar to the healthy control cohort (Fig. 5a), total copper and labile Cu2+ did not correlate (Fig. 6c). Likewise, CP and labile Cu2+ showed no association (Fig. 6d), while total copper and CP showed a strong correlation (r = 0.89, p < 0.001) as described before (Fig. 6e)8. During WD the incorporation of Cu2+ in apo-CP in hepatocytes is impaired due to loss of function of ATPase copper transporting beta (ATP7B), responsible for shuttling absorbed Cu2+ into the Golgi. Consequently, serum CP and CP-bound Cu2+ levels in serum are low whereas non-CP bound Cu2+ is still excreted into the blood stream, resulting in an increase of loosely bound and labile Cu2+ in serum. Accordingly, serum Cu2+ species are suggested to represent the harmful Cu2+ serum pool leading to neurological disorders observed in WD, whereas total copper seems to be a poor marker of disease severity44. Increased loosely bound8,20 or exchangeable Cu2+45 levels in serum of WD patients compared to healthy controls were reported before, suggesting it to be a more specific biomarker for disease diagnosis, status, or monitoring therapy success8,9,45. This is also consistent with the observations with the present fluorometric assay. The analysis of labile Cu2+ with this method could be used in the future to monitor the progress of medical therapy more easily. Its use for this purpose will need to be further validated in future studies with a range of different therapies for WD.
Labile Cu2+ in Wilson’s disease patients before and after therapy. Labile Cu2+ (a) and total copper (b) concentrations in serum of a group of WD patients (N = 19) before and after medical therapy. Labile Cu2+ concentrations in serum were compared to total copper (c) and ceruloplasmin levels (d) before and after therapy. Correlation of total copper and ceruloplasmin concentrations in serum of patients before and after treatment (e). Data are presented as means + SD, and p values are indicated (unpaired t-test (a,b)) and as scatter plots, including Spearman correlation coefficients (r) and p-values (c–e).
Conclusion
This study presents a newly developed direct fluorometric assay based on a novel Cu2+-binding fluorescent probe. The high-throughput assay requires only 5 µl of serum to quantify labile Cu2+ content in serum and can measure 76 serum samples in less than 4 h, making it a suitable platform for determining the labile Cu2+ concentration in serum samples from larger cohorts. Therefore, the assay may be used for future clinical applications, such as monitoring the copper status of WD patients during treatment. Furthermore, this study shows that the labile Cu2+ species most likely represents a separate serum Cu2+ pool and not just a subfraction of total serum Cu2+. Future studies should further investigate the role of this highly available Cu2+ pool in the development and severity of diseases with impaired Cu2+ homeostasis.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Change history
17 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-59455-z
References
Sensi, S. L., Granzotto, A., Siotto, M. & Squitti, R. Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharmacol. Sci. 39, 1049–1063. https://doi.org/10.1016/j.tips.2018.10.001 (2018).
Bornhorst, J., Kipp, A. P., Haase, H., Meyer, S. & Schwerdtle, T. The crux of inept biomarkers for risks and benefits of trace elements. TrAC Trends Anal. Chem. 104, 183–190. https://doi.org/10.1016/j.trac.2017.11.007 (2018).
Linder, M. C. Ceruloplasmin and other copper binding components of blood plasma and their functions: An update. Metallomics Integr. Biomet. Sci. 8, 887–905. https://doi.org/10.1039/c6mt00103c (2016).
Kirsipuu, T. et al. Copper(II)-binding equilibria in human blood. Sci. Rep. 10, 5686. https://doi.org/10.1038/s41598-020-62560-4 (2020).
Gioilli, B. D. et al. Secretion and uptake of copper via a small copper carrier in blood fluid. Metallomics Integr. Biomet. Sci. https://doi.org/10.1093/mtomcs/mfac006 (2022).
Catalani, S. et al. Free copper in serum: An analytical challenge and its possible applications. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 45, 176–180. https://doi.org/10.1016/j.jtemb.2017.11.006 (2018).
Witt, B., Schaumloffel, D. & Schwerdtle, T. Subcellular localization of copper-cellular bioimaging with focus on neurological disorders. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21072341 (2020).
McMillin, G. A., Travis, J. J. & Hunt, J. W. Direct measurement of free copper in serum or plasma ultrafiltrate. Am. J. Clin. Pathol. 131, 160–165. https://doi.org/10.1309/ajcp7z9kbfinvgyf (2009).
Poujois, A. et al. Exchangeable copper: A reflection of the neurological severity in Wilson’s disease. Eur. J. Neurol. 24, 154–160. https://doi.org/10.1111/ene.13171 (2017).
Ge, E. J. et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 22, 102–113. https://doi.org/10.1038/s41568-021-00417-2 (2022).
Squitti, R. et al. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer’s disease. J. Alzheimers Dis. 38, 809–822. https://doi.org/10.3233/JAD-131247 (2014).
Squitti, R. et al. Non-ceruloplasmin copper distincts subtypes in Alzheimer’s disease: A genetic study of ATP7B frequency. Mol. Neurobiol. 54, 671–681. https://doi.org/10.1007/s12035-015-9664-6 (2017).
Squitti, R., Siotto, M., Cassetta, E., El Idrissi Imane, G. & Colabufo Nicola, A. Clinical Chemistry and Laboratory Medicine (CCLM). Vol. 55. 1360 (2017).
Kumar, A., Sharma, P., Prasad, R. & Pal, A. An urgent need to assess safe levels of inorganic copper in nutritional supplements/parenteral nutrition for subset of Alzheimer’s disease patients. Neurotoxicology 73, 168–174. https://doi.org/10.1016/j.neuro.2019.04.001 (2019).
Squitti, R. et al. Non-ceruloplasmin copper identifies a subtype of Alzheimer’s disease (CuAD): Characterization of the cognitive profile and case of a CuAD patient carrying an RGS7 stop-loss variant. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24076377 (2023).
Kumar, A., Gupta, S., Sharma, P., Prasad, R. & Pal, A. In silico method for identification of novel copper and iron metabolism proteins in various neurodegenerative disorders. Neurotoxicology 73, 50–57. https://doi.org/10.1016/j.neuro.2019.02.020 (2019).
Walshe, J. M. Wilson’s disease: The importance of measuring serum caeruloplasmin non-immunologically. Ann. Clin. Biochem. 40, 115–121. https://doi.org/10.1258/000456303763046021 (2003).
Twomey, P. J., Viljoen, A., House, I. M., Reynolds, T. M. & Wierzbicki, A. S. Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin. Chem. 51, 1558–1559. https://doi.org/10.1373/clinchem.2005.052688 (2005).
Quarles, C. D. et al. LC-ICP-MS method for the determination of “extractable copper” in serum. Metallomics Integr. Biomet. Sci. 12, 1348–1355. https://doi.org/10.1039/D0MT00132E (2020).
del Castillo Busto, M. E., Cuello-Nunez, S., Ward-Deitrich, C., Morley, T. & Goenaga-Infante, H. A fit-for-purpose copper speciation method for the determination of exchangeable copper relevant to Wilson’s disease. Anal. Bioanal. Chem. https://doi.org/10.1007/s00216-021-03517-y (2021).
Tuchtenhagen, M., Stiboller, M., Witt, B. & Schwerdtle, T. A novel approach for the determination of exchangeable copper in serum using protein precipitation. J. Anal. At. Spectrom. https://doi.org/10.1039/D2JA00355D (2023).
Inagaki, K. et al. Speciation of protein-binding zinc and copper in human blood serum by chelating resin pre-treatment and inductively coupled plasma mass spectrometry. Analyst 125, 197–203. https://doi.org/10.1039/a907088e (2000).
Venelinov, T. I., Davies, I. M. & Beattie, J. H. Dialysis-Chelex method for determination of exchangeable copper in human plasma. Anal. Bioanal. Chem. 379, 777–780. https://doi.org/10.1007/s00216-004-2529-x (2004).
Bohrer, D. et al. Comparison of ultrafiltration and solid phase extraction for the separation of free and protein-bound serum copper for the Wilson's disease diagnosis. Clin. Chim. Acta Int. J. Clin. Chem. 345, 113–121. https://doi.org/10.1016/j.cccn.2004.03.001 (2004).
El Balkhi, S. et al. Determination of ultrafiltrable and exchangeable copper in plasma: Stability and reference values in healthy subjects. Anal. Bioanal. Chem. 394, 1477–1484. https://doi.org/10.1007/s00216-009-2809-6 (2009).
Alker, W., Schwerdtle, T., Schomburg, L. & Haase, H. A zinpyr-1-based fluorimetric microassay for free zinc in human serum. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20164006 (2019).
Williamson, D. J., Fascione, M. A., Webb, M. E. & Turnbull, W. B. Efficient n-terminal labeling of proteins by use of sortase. Angew. Chem. Int. Ed. 51, 9377–9380. https://doi.org/10.1002/anie.201204538 (2012).
Young, T. R. et al. A set of robust fluorescent peptide probes for quantification of Cu(II) binding affinities in the micromolar to femtomolar range. Metallomics Integr. Biomet. Sci. 7, 567–578. https://doi.org/10.1039/c4mt00301b (2015).
Xiao, Z. & Wedd, A. G. The challenges of determining metal–protein affinities. Nat. Prod. Rep. 27, 768–789. https://doi.org/10.1039/B906690J (2010).
Sokołowska, M. & Bal, W. Cu(II) complexation by “non-coordinating” N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES buffer). J. Inorg. Biochem. 99, 1653–1660. https://doi.org/10.1016/j.jinorgbio.2005.05.007 (2005).
Einer, C. et al. A high-calorie diet aggravates mitochondrial dysfunction and triggers severe liver damage in Wilson disease rats. Cell. Mol. Gastroenterol. Hepatol. 7, 571–596. https://doi.org/10.1016/j.jcmgh.2018.12.005 (2019).
Grynkiewicz, G., Poenie, M. & Tsien, R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985).
Heller, R. A. et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 38, 101764. https://doi.org/10.1016/j.redox.2020.101764 (2021).
Maares, M. et al. Free zinc as a predictive marker for COVID-19 mortality risk. Nutrients 14, 1407. https://doi.org/10.3390/nu14071407 (2022).
Carter, K. P., Young, A. M. & Palmer, A. E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 114, 4564–4601. https://doi.org/10.1021/cr400546e (2014).
Lichtman, J. W. & Conchello, J. A. Fluorescence microscopy. Nat. Methods 2, 910–919. https://doi.org/10.1038/nmeth817 (2005).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy (Springer, 2006).
Bocca, B. et al. Nickel quantification in serum by a validated sector-field inductively coupled plasma mass spectrometry method: Assessment of tentative reference values for an Italian population. Rapid Commun. Mass Spectrom. 20, 3289–3294. https://doi.org/10.1002/rcm.2731 (2006).
Krezel, A. & Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 11, 1049–1062. https://doi.org/10.1007/s00775-006-0150-5 (2006).
Dineley, K. E., Malaiyandi, L. M. & Reynolds, I. J. A reevaluation of neuronal zinc measurements: Artifacts associated with high intracellular dye concentration. Mol. Pharmacol. 62, 618–627. https://doi.org/10.1124/mol.62.3.618 (2002).
Konz, T. et al. Sex-specific associations of blood-based nutrient profiling with body composition in the elderly. Front. Physiol. https://doi.org/10.3389/fphys.2018.01935 (2019).
Piacenza, F. et al. Age, sex and BMI influence on copper, zinc and their major serum carrier proteins in a large European population including nonagenarian offspring from MARK-AGE study. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glab134 (2021).
Mohr, I. & Weiss, K. H. Current anti-copper therapies in management of Wilson disease. Ann. Transl. Med. 7, S69–S69. https://doi.org/10.21037/atm.2019.02.48 (2019).
Stremmel, W. & Weiskirchen, R. Therapeutic strategies in Wilson disease: Pathophysiology and mode of action. Ann. Transl. Med. 9, 732–732. https://doi.org/10.21037/atm-20-3090 (2021).
El Balkhi, S. et al. Relative exchangeable copper: A new highly sensitive and highly specific biomarker for Wilson's disease diagnosis. Clin. Chim. Acta Int. J. Clin. Chem. 412, 2254–2260. https://doi.org/10.1016/j.cca.2011.08.019 (2011).
Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG), Research Unit FOR-2558 TraceAge-DFG Research Unit on Interactions of essential trace elements in healthy and diseased elderly, Potsdam-Berlin-Jena (HA 4318/4-2, Scho 849/6-2). M.M. is funded by DFG (MA 9681/1-1). M.K.-K. is funded by the Dahlem Research School (Elsa-Neumann-Stipendium für Promovierende, Τ70011).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conception of the study: M.M., H.H.; analysis: A.H., C.S., M.K.-K., J.H., M.M., I. M.; interpretation of data: M.M., H.H., resources: H.H., L.S., H.Z., R.D.S., U.M. writing—original draft: M.M., M.K.-K.; writing—review and editing: H.H., C.K., I.M., L.S., H.Z., R.D.S., U.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in Figure 4c. The values for ‘Storage temperature’, displayed on the X-axis, were inadvertently changed into the mean values of the depicted bars. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maares, M., Haupt, A., Schüßler, C. et al. A fluorometric assay to determine labile copper(II) ions in serum. Sci Rep 13, 12807 (2023). https://doi.org/10.1038/s41598-023-39841-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39841-9
This article is cited by
-
Symmetrical Ligand’s Fabricated Porous Silicon Surface Based Photoluminescence Sensor for Metal Detection and Entrapment
Journal of Fluorescence (2024)
-
Ratiometric Fluorescence and Chromogenic Probe for Trace Detection of Selected Transition Metals
Journal of Fluorescence (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.