Abstract
Inborn defects in DNA repair are associated with complex developmental disorders whose causal mechanisms are poorly understood. Using an in vivo biotinylation tagging approach in mice, we show that the nucleotide excision repair (NER) structure-specific endonuclease ERCC1–XPF complex interacts with the insulator binding protein CTCF, the cohesin subunits SMC1A and SMC3 and with MBD2; the factors co-localize with ATRX at the promoters and control regions (ICRs) of imprinted genes during postnatal hepatic development. Loss of Ercc1 or exposure to MMC triggers the localization of CTCF to heterochromatin, the dissociation of the CTCF–cohesin complex and ATRX from promoters and ICRs, altered histone marks and the aberrant developmental expression of imprinted genes without altering DNA methylation. We propose that ERCC1–XPF cooperates with CTCF and cohesin to facilitate the developmental silencing of imprinted genes and that persistent DNA damage triggers chromatin changes that affect gene expression programs associated with NER disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Harper, J. W. & Elledge, S. J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007).
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001).
Gregg, S. Q., Robinson, A. R. & Niedernhofer, L. J. Physiological consequences of defects in ERCC1–XPF DNA repair endonuclease. DNA Rep. 10, 781–791 (2011).
Marteijn, J. A., Lans, H., Vermeulen, W. & Hoeijmakers, J. H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 15, 465–481 (2014).
van Duin, M. et al. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell 44, 913–923 (1986).
Sijbers, A. M. et al. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell 86, 811–822 (1996).
Hoy, C. A., Thompson, L. H., Mooney, C. L. & Salazar, E. P. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 45, 1737–1743 (1985).
Klein Douwel, D. et al. XPF-ERCC1 acts in unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4. Mol. Cell 54, 460–471 (2014).
Niedernhofer, L. J. et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 24, 5776–5787 (2004).
Bergstralh, D. T. & Sekelsky, J. Interstrand crosslink repair: can XPF-ERCC1 be let off the hook? Trends Genet. 24, 70–76 (2008).
Kamileri, I., Karakasilioti, I. & Garinis, G. A. Nucleotide excision repair: new tricks with old bricks. Trends Genet. 28, 566–573 (2012).
Bootsma, D., Kraemer, K. H., Cleaver, J. E. & Hoeijmakers, J. H. J. in The Genetic Basis of Human Cancer (eds Vogelstein, B. & Kinzler, K. W.) 245–274 (McGraw-Hill, 1998).
Bootsma, D., Kraemer, K. H., Cleaver, J. E. & Hoeijmakers, J. H. J. The Metabolic and Molecular Basis of Inherited Disease (McGraw-Hill, 2001).
Itin, P. H., Sarasin, A. & Pittelkow, M. R. Trichothiodystrophy: update on the sulfur-deficient brittle hair syndromes. J. Am. Acad. Dermatol. 44, 891–920 (2001).
Garinis, G. A., van der Horst, G. T., Vijg, J. & Hoeijmakers, J. H. DNA damage and ageing: new-age ideas for an age-old problem. Nat. Cell Biol. 10, 1241–1247 (2008).
Cleaver, J. E., Thompson, L. H., Richardson, A. S. & States, J. C. A summary of mutations in the UV-sensitive disorders: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. Hum. Mutat. 14, 9–22 (1999).
Jaspers, N. G. et al. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am. J. Hum. Genet. 80, 457–466 (2007).
Bootsma, D., Kraemer, K. H., Cleaver, J. E. & Hoeijmakers, J. H. J. in The Metabolic and Molecular Basis of Inherited Disease Vol. 1 (eds Scriver, C. R. et al.) 677–703 (McGraw-Hill, 2001).
Le May, N. et al. NER factors are recruited to active promoters and facilitate chromatin modification for transcription in the absence of exogenous genotoxic attack. Mol. Cell 38, 54–66 (2010).
Le May, N., Egly, J. M. & Coin, F. True lies: the double life of the nucleotide excision repair factors in transcription and DNA repair. J. Nucleic Acids 2010, 616342 (2010).
Le May, N., Fradin, D., Iltis, I., Bougneres, P. & Egly, J. M. XPG and XPF endonucleases trigger chromatin looping and DNA demethylation for accurate expression of activated genes. Mol. Cell 47, 622–632 (2012).
Fong, Y. W. et al. A DNA repair complex functions as an Oct4/Sox2 coactivator in embryonic stem cells. Cell 147, 120–131 (2011).
Kamileri, I. et al. Defective transcription initiation causes postnatal growth failure in a mouse model of nucleotide excision repair (NER) progeria. Proc. Natl Acad. Sci. USA 109, 2995–3000 (2012).
Beckett, D., Kovaleva, E. & Schatz, P. J. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8, 921–929 (1999).
O’Gorman, S., Dagenais, N. A., Qian, M. & Marchuk, Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl Acad. Sci. USA 94, 14602–14607 (1997).
Katsantoni, E. Z. et al. Ubiquitous expression of the rtTA2S-M2 inducible system in transgenic mice driven by the human hnRNPA2B1/CBX3 CpG island. BMC Dev. Biol. 7, 108 (2007).
Tian, M., Shinkura, R., Shinkura, N. & Alt, F. W. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol. Cell. Biol. 24, 1200–1205 (2004).
McWhir, J., Selfridge, J., Harrison, D. J., Squires, S. & Melton, D. W. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat. Genet. 5, 217–224 (1993).
Selfridge, J., Hsia, K. T., Redhead, N. J. & Melton, D. W. Correction of liver dysfunction in DNA repair-deficient mice with an ERCC1 transgene. Nucleic Acids Res. 29, 4541–4550 (2001).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
Bonora, G., Plath, K. & Denholtz, M. A mechanistic link between gene regulation and genome architecture in mammalian development. Curr. Opin. Genet. Dev. 27, 92–101 (2014).
Kernohan, K. D. et al. ATRX partners with cohesin and MeCP2 and contributes to developmental silencing of imprinted genes in the brain. Dev. Cell 18, 191–202 (2010).
Phillips, J. E. & Corces, V. G. CTCF: master weaver of the genome. Cell 137, 1194–1211 (2009).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
Lewis, A. & Murrell, A. Genomic imprinting: CTCF protects the boundaries. Curr. Biol. 14, R284–R286 (2004).
Nativio, R. et al. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet. 5, e1000739 (2009).
Barlow, D. P. & Bartolomei, M. S. Genomic imprinting in mammals. Cold Spring Harb. Perspect. Biol. 6, 6:a018382 (2014).
Lui, J. C., Finkielstain, G. P., Barnes, K. M. & Baron, J. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R189–R196 (2008).
Schumacher, B. et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS Genet. 4, e1000161 (2008).
van der Pluijm, I. et al. Impaired genome maintenance suppresses the growth hormone–insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 5, e2 (2006).
de Boer, J. et al. Premature aging in mice deficient in DNA repair and transcription. Science 296, 1276–1279 (2002).
Charalambous, M. et al. Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an Igf2-independent mechanism. Proc. Natl Acad. Sci. USA 100, 8292–8297 (2003).
Leighton, P. A., Ingram, R. S., Eggenschwiler, J., Efstratiadis, A. & Tilghman, S. M. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 375, 34–39 (1995).
Li, L. et al. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 284, 330–333 (1999).
Moon, Y. S. et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 22, 5585–5592 (2002).
Karakasilioti, I. et al. DNA damage triggers a chronic autoinflammatory response, leading to fat depletion in NER progeria. Cell Metab. 18, 403–415 (2013).
Plasschaert, R. N. & Bartolomei, M. S. Genomic imprinting in development, growth, behavior and stem cells. Development 141, 1805–1813 (2014).
Kurukuti, S. et al. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl Acad. Sci. USA 103, 10684–10689 (2006).
Murrell, A., Heeson, S. & Reik, W. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat. Genet. 36, 889–893 (2004).
Snapp, R. R. et al. Spatial organization of fibroblast nuclear chromocenters: component tree analysis. J. Anat. 223, 255–261 (2013).
Westerveld, A. et al. Molecular cloning of a human DNA repair gene. Nature 310, 425–429 (1984).
Hoy, C. A., Thompson, L. H., Mopney, C. L. & Salazar, E. P. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 45, 1737–1743 (1985).
Garinis, G. A. et al. Transcriptome analysis reveals cyclobutane pyrimidine dimers as a major source of UV-induced DNA breaks. EMBO J. 24, 3952–3962 (2005).
Ding, J., Miao, Z. H., Meng, L. H. & Geng, M. Y. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol. Sci. 27, 338–344 (2006).
Gregor, A. et al. De novo mutations in the genome organizer CTCF cause intellectual disability. Am. J. Hum. Genet. 93, 124–131 (2013).
Boyle, M. I., Jespersgaard, C., Brondum-Nielsen, K., Bisgaard, A. M. & Tumer, Z. Cornelia de Lange syndrome. Clin. Genet. 88, 1–12 (2015).
Gibbons, R. Alpha thalassaemia-mental retardation, X linked. Orphan. J. Rare Dis. 1, 15 (2006).
Dowen, J. M. et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159, 374–387 (2014).
Ling, J. Q. et al. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312, 269–272 (2006).
Niedernhofer, L. J. Nucleotide excision repair deficient mouse models and neurological disease. DNA Repair (Amst.) 7, 1180–1189 (2008).
Shiomi, N. et al. Identification of the XPG region that causes the onset of Cockayne syndrome by using Xpg mutant mice generated by the cDNA-mediated knock-in method. Mol. Cell Biol. 24, 3712–3719 (2004).
Tian, M., Shinkura, R., Shinkura, N. & Alt, F. W. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol. Cell Biol. 24, 1200–1205 (2004).
van der Pluijm, I. et al. Impaired genome maintenance suppresses the growth hormone–insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 5, e2 (2007).
McNeil, E. M. & Melton, D. W. DNA repair endonuclease ERCC1–XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 40, 9990–10004 (2012).
Antoniou, M. et al. Transgenes encompassing dual-promoter CpG islands from the human TBP and HNRPA2B1 loci are resistant to heterochromatin-mediated silencing. Genomics 82, 269–279 (2003).
Aivaliotis, M. et al. Large-scale identification of N-terminal peptides in the halophilic archaea Halobacterium salinarum and Natronomonas pharaonis. J. Proteome Res. 6, 2195–2204 (2007).
Rappsilber, J., Ryder, U., Lamond, A. I. & Mann, M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 12, 1231–1245 (2002).
Szklarczyk, D. et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 (2011).
Vizcaino, J. A. et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 (2013).
Acknowledgements
The THALIS ESPA 2007–2013 ‘GenAge’ (MIS380228) and ‘miREG’ (MIS380247), the ARISTEIA ‘TagNER’ (45) and ‘Epilogeas’ (3446), the FP7 Marie Curie ITN ‘aDDRess (GA316390), ‘CodeAge’ (GA316354), ‘Marriage’ (GA316964) and ‘Chromatin3D (GA622934), and the Horizon 2020 ERC Consolidator grant ‘DeFiNER’ (GA64663) supported this work. G.A.G. was supported by the EMBO Young Investigator programme. I.K. was supported by the Maria-Michail Manassakis fellowship. We thank M. Fousteri for providing the reagents for the unscheduled DNA synthesis assay and the Fanconi Anemia Research Fund (FA Cell Repository and the FA Antibody Project) for anti-FANCA and anti-FANCD2 antibodies and corresponding mutant MEFs.
Author information
Authors and Affiliations
Contributions
G.C., Z.A., T.A.-P., A.I., M.T., M.A., I.K. and T.K. performed the experiments and analysed data. T.K., G.L.P. and J.S. generated new reagents. G.A.G. interpreted data and wrote the manuscript. All relevant data are available from the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
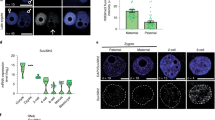
Supplementary Figure 1 Ablation of Ercc1 gene triggers the aberrant silencing of imprinted genes during postnatal hepatic development.
(A) Schematic representation of transgenic mice expressing the BirA biotin ligase transgene and anti-HA immunoblot showing expression of the BirA biotin ligase protein in different tissues of 2-month old BirA transgenic animals (as indicated). (B) Streptavidin pull-downs in nuclear or chromatin extracts under native (micrococcal nuclease digested) conditions derived from primary bXPF MEFs expressing the BirA transgene or the BirA transgenic animals (as indicated) and analysed by Western blotting for CTCF. (C) Over-represented biological processes derived from the significantly aberrantly expressed imprinted genes in P15 Ercc1−/− compared to age-matched wt livers; p : -log of P-value which is calculated by Fisher’s exact test right-tailed. Red dotted line marks the threshold of significance at 0.05. (D) qPCR mRNA levels of imprinted genes in P15 Ercc1−/− spleen, kidney, white adipose tissue (WAT), pancreas and cerebellum (as indicated; n = 3 biological replicates each representing a pool of 4-5 tissues/genotype). Red dotted line: wt mRNA levels. Error bars indicate s.d.; two tailed t-test. Statistical source data are provided in Supplementary Table 6.
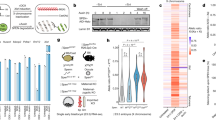
Supplementary Figure 2 DNA methylation at the Igf2, Peg3, Dlk1 and Peg3 proximal promoter regions in Ercc1−/− livers.
(A) ChIP-seq profiles marking the recruitment of CTCF and RNAPII (Pol2) at the H19, Dlk1, Peg3 and Grb10 promoter and ICRs/DMR regions in livers (liv) or mouse embryonic fibroblasts (MEFs) as indicated. Arrow heads mark the PCR amplified ICR or promoter (prom) regions (as indicated). (B–G) Schematic representation of the% of DNA methylation at the Peg3 (promoter), Grb10 (CTCF-peak), Igf2 (promoter 2 and 3), Grb10 (promoter) and Meg3 (ICR) regions (as indicated) in P15 Ercc1−/− and wt livers. Black circles: methylated cytosine; open circles: unmethylated cytosine.
Supplementary Figure 3 Dissociation of the CTCF-cohesin complex and MBD2 from the promoters and ICRs of imprinted genes in Ercc1−/− livers.
(A) bXPF, CTCF, SMC1A, SMC3, MBD2 and ATRX ChIP signals expressed as fold enrichment over those obtained with BirA (for bXPF) or control antibody (IgG) at the Igf2, Peg3, Dlk1 and Grb10 promoters in P15 wt mouse livers (as indicated; n = 3 biological replicates each representing a pool of 4–5 livers). Error bars indicate s.e.m. among replicates (n ≥ 3). (B) ChIP signals shown as % of input of CTCF, SMC1A, SMC3, MBD2 and ATRX at the Igf2, Peg3, Meg3 and Grb10 ICR regions and CTCF negative (-) regions (as indicated; n = 3 biological replicates each representing a pool of 4–5 livers). Error bars indicate s.e.m. (C) CTCF, SMC1A, SMC3, MBD2 and ATRX ChIP signals normalized against their respective control antibody (IgG) and expressed as fold enrichment over the corresponding ChIP signals obtained for wt mouse livers at the Dlk1/Meg3 and Grb10 promoters and ICRs (as indicated; n = 3 biological replicates each representing a pool of 4–5 livers). Error bars indicate s.e.m., ∗:P ≤ 0.05; two-tailed t-test. Statistical source data are provided in Supplementary Table 6.
Supplementary Figure 4 Persistent DNA ICLs trigger aberrant CTCF and ATRX localization in Ercc1−/− and MMC-treated MEFs.
(A) Immunofluorescence detection of CTCF in Csbm/m primary mouse embryonic fibroblasts (MEFs; 20 fields analysed from 3 biological replicates). (B) Equal amount of nuclear extracts from wt, MMC-treated and Ercc1−/− MEFs analysed by Western blotting for CTCF, SMC1A and TBP. (C) Immunocolocalization of CTCF and HP1a in MMC-treated and untreated P4 MEFs. (D) Immunofluorescence detection of ATRX in Ercc1−/−, Csbm/m, Xpc−/− and Xpa−/− primary mouse embryonic fibroblasts (MEFs; 20 fields analysed from 3 biological replicates). Note the distinctive accumulation of ATRX to heterochromatin in Ercc1−/− MEFs. (E) Immunofluorescence detection of SMC1A in primary Csbm/m MEFs. (F) Immunofluorescence detection of SMC3 in wt, Ercc1−/−, Csbm/m, Xpc−/− and Xpa−/−primary MEFs (as indicated; 20 fields analysed from 3 biological replicates). (G–H). Immunofluorescence detection of CTCF and ATRX in primary wt and Ercc1−/− hepatocytes; note the distinctive translocation of CTCF and accumulation of ATRX to heterochromatin Ercc1−/− hepatocytes. (I). Immunofluorescence detection of γH2aX and nucleolin in wt and Ercc1−/− MEFs (upper panel) and in MMC-treated and untreated control (ctrl) MEFs (lower panel); the graph depicts the average number of γH2aX-positive stained cells in wt and Ercc1−/− MEFs or wt MEFs exposed to MMC from 20 fields analysed representing n = 3 biological replicates∗: P-value ≤ 0.05. (J) Immunocolocalization of CTCF and γH2aX in MMC-treated and untreated P4 MEFs. Scale bars, 5 μm. Statistical source data are provided in Supplementary Table 6.
Supplementary Figure 5 Persistent DNA ICLs trigger aberrant CTCF and ATRX localization in Ercc1−/− and MMC-treated MEFs.
(A) Immunofluorescence detection of CTCF in serum starved (SS) MEFs exposed to MMC (as indicated; 20 fields analysed from 3 biological replicates). (B) Immunofluorescence detection of ATRX in primary MEFs exposed to MMC, UV and H2O2; Ctr: untreated MEFs. Note the distinctive accumulation of ATRX to heterochromatin in MMC-treated MEFs. (C) Immunocolocalization of CTCF and ATRX in MMC-treated primary P4 MEFs. (D) Co-immunoprecipitation experiments using αCTCF in nuclear extracts from P15 livers analysed by Western blotting for FANCA or FANCD2. (E) Immunofluorescence detection of CTCF in Fanca−/− and Fancd2−/− MEFs (as indicated; 20 fields analysed from 3 biological replicates). Scale bars, 5 μm.
Supplementary Figure 6 Persistent DNA ICLs trigger the dissociation of the CTCF-cohesin complex and MBD2 from promoters and ICRs.
(A). Quantitative (q) PCR mRNA levels of UV-responsive and imprinted genes in primary mouse dermal fibroblasts exposed to 0.6 and 4 J m−2 of UVC irradiation (as indicated), n = 3 biological replicates/dose. Error bars indicate s.d. (B). qPCR mRNA levels of H2O2-responsive and imprinted genes in primary mouse dermal fibroblasts treated with 10 μM or 50 μM of H2O2 (as indicated), n = 3 biological replicates/dose. Error bars indicate s.d. (C) ChIP signals of CTCF, SMC1A and SMC3 (as indicated) at the H19, Peg3, Dlk1 and Grb10 promoters in primary MEFs exposed to UV, H2O2 and MMC or to MMC and ATM or ATR inhibitors (ATMi, ATRi as indicated). ChIP signals from treated MEFs were normalized to respective control antibody (IgG) which were set as 1 (red dotted line) and expressed as fold enrichment over those obtained from untreated MEFs, n = 3 biological replicates. Error bars indicate s.d.; two-sided t-test. Statistical source data are provided in Supplementary Table 6.
Supplementary Figure 7 Persistent DNA ICLs trigger changes in histone marks associated with aberrant postnatal silencing.
(A) Allele-specific ChIP signals of CTCF, SMC1A and SMC3 (as indicated) at the H19 ICR in MMC-treated C57BL/6/SPRET/Eij MEFs (as indicated). ChIP signals expressed as fold enrichment over those obtained with control antibody (IgG). Error bars indicate s.d. among biological replicates; (n = 3 biological replicates). (B) ChIP signals of repressive H3K27me and H3K9me3 histone marks at the H19, Peg3, Dlk1 and Grb10 promoters in primary MEFs exposed to MMC, UV or H2O2 (as indicated) and in MMC-treated MEFs treated with ATMi or ATRi (as indicated). ChIP signals are shown as in Fig. 5c. To test for significance, ChIP signals of ATMi and ATRi-treated MEFs are compared against those of MMC-treated MEFs; two sided t-test. (C–D). ChIP signals of activating H3K9Ac and H3K4me3 histone marks at the H19/Igf2, Peg3, Meg3/Dlk1 and Grb10 ICRs and promoters in primary MEFs exposed to MMC, UV or H2O2 (as indicated) and in MMC-treated MEFs treated with ATMi or ATRi (as indicated; n = 3 biological replicates). ChIP signals are shown as in Fig. 5c. To test for significance, ChIP signals of MMC-treated MEFs are compared against untreated cells; ChIP signals of ATMi and ATRi-treated MEFs are compared against those of MMC-treated MEFs. ∗∗:P ≤ 0.01, Error bars indicate s.d.; two-sided t-test. Statistical source data are provided in Supplementary Table 6.
Supplementary information
Supplementary Information
Supplementary Information (PDF 9089 kb)
Supplementary Table 1
Supplementary Information (XLSX 207 kb)
Supplementary Table 2
Supplementary Information (XLSX 26 kb)
Supplementary Table 3
Supplementary Information (XLSX 22 kb)
Supplementary Table 4
Supplementary Information (XLSX 14 kb)
Supplementary Table 5
Supplementary Information (XLSX 12 kb)
Supplementary Table 6
Supplementary Information (XLSX 100 kb)
Rights and permissions
About this article
Cite this article
Chatzinikolaou, G., Apostolou, Z., Aid-Pavlidis, T. et al. ERCC1–XPF cooperates with CTCF and cohesin to facilitate the developmental silencing of imprinted genes. Nat Cell Biol 19, 421–432 (2017). https://doi.org/10.1038/ncb3499
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3499