Abstract
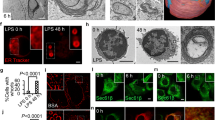
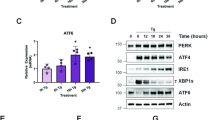
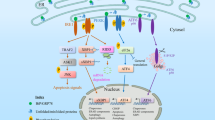
Activation of Toll-like receptors (TLRs) induces the endoplasmic reticulum (ER) unfolded protein response (UPR) to accommodate essential protein translation1,2. However, despite increased levels of phosphorylated eIF2α (p-eIF2α), a TLR–TRIF-dependent pathway assures that the cells avoid CHOP induction, apoptosis and translational suppression of critical proteins3. As p-eIF2α decreases the functional interaction of eIF2 with eIF2B, a guanine nucleotide exchange factor (GEF), we explored the hypothesis that TLR–TRIF signalling activates eIF2B GEF activity to counteract the effects of p-eIF2α. We now show that TLR–TRIF signalling activates eIF2B GEF through PP2A-mediated serine dephosphorylation of the eIF2B ɛ-subunit. PP2A itself is activated by decreased Src-family-kinase-induced tyrosine phosphorylation of its catalytic subunit. Each of these processes is required for TLR–TRIF-mediated CHOP suppression in ER-stressed cells in vitro and in vivo. Thus, in the setting of prolonged, physiologic ER stress, a unique TLR–TRIF-dependent translational control pathway enables cells to carry out essential protein synthesis and avoid CHOP-induced apoptosis while still benefiting from the protective arms of the UPR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Todd, D. J., Lee, A. H. & Glimcher, L. H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 8, 663–674 (2008).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Woo, C. W. et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat. Cell Biol. 11, 1473–1480 (2009).
Ron, D. Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 110, 1383–1388 (2002).
Tabas, I. & Ron, D. Molecular mechanisms integrating pathways of endoplasmic reticulum stress-induced apoptosis. Nat. Cell Biol. 13, 184–190 (2011).
Oyadomari, S. & Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death. Differ. 11, 381–389 (2004).
Krishnamoorthy, T., Pavitt, G. D., Zhang, F., Dever, T. E. & Hinnebusch, A. G. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell Biol. 21, 5018–5030 (2001).
Welsh, G. I., Miller, C. M., Loughlin, A. J., Price, N. T. & Proud, C. G. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 421, 125–130 (1998).
Wang, X. & Proud, C. G. A novel mechanism for the control of translation initiation by amino acids, mediated by phosphorylation of eukaryotic initiation factor 2B. Mol. Cell Biol. 28, 1429–1442 (2008).
Hardt, S. E., Tomita, H., Katus, H. A. & Sadoshima, J. Phosphorylation of eukaryotic translation initiation factor 2Bε by glycogen synthase kinase-3β regulates β-adrenergic cardiac myocyte hypertrophy. Circ. Res. 94, 926–935 (2004).
Fang, X. et al. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl Acad. Sci. USA 97, 11960–11965 (2000).
Chung, H., Nairn, A. C., Murata, K. & Brautigan, D. L. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favors association with the α 4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry 38, 10371–10376 (1999).
Evans, D. R., Myles, T., Hofsteenge, J. & Hemmings, B. A. Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-negative mutant forms. J. Biol. Chem. 274, 24038–24046 (1999).
Santa-Coloma, T. A. Anp32e (Cpd1) and related protein phosphatase 2 inhibitors. Cerebellum 2, 310–320 (2003).
Nanahoshi, M. et al. α4 protein as a common regulator of type 2A-related serine/threonine protein phosphatases. FEBS Lett. 446, 108–112 (1999).
Chen, J., Martin, B. L. & Brautigan, D. L. Regulation of protein serine–threonine phosphatase type-2A by tyrosine phosphorylation. Science 257, 1261–1264 (1992).
Hu, X. et al. Src kinase up-regulates the ERK cascade through inactivation of protein phosphatase 2A following cerebral ischemia. BMC. Neurosci. 10, 74 (2009).
Barisic, S., Schmidt, C., Walczak, H. & Kulms, D. Tyrosine phosphatase inhibition triggers sustained canonical serine-dependent NFκB activation via Src-dependent blockade of PP2A. Biochem. Pharmacol. 80, 439–447 (2010).
Brown, M. T. & Cooper, J. A. Regulation, substrates and functions of src. Biochim. Biophys. Acta 1287, 121–149 (1996).
Liu, X. et al. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene 8, 1119–1126 (1993).
Lu, B. et al. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 457, 741–744 (2009).
Encinas, M. et al. c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J. Neurosci. 21, 1464–1472 (2001).
Mayhew, D. L., Hornberger, T. A., Lincoln, H. C. & Bamman, M. M. Eukaryotic initiation factor 2Bε induces cap-dependent translation and skeletal muscle hypertrophy. J. Physiol. 589, 3023–3037 (2011).
Potter, M. W., Shah, S. A., Elbirt, K. K. & Callery, M. P. Endotoxin (LPS) stimulates 4E-BP1/PHAS-I phosphorylation in macrophages. J. Surg. Res. 97, 54–59 (2001).
Lee, J. Y. et al. The regulation of the expression of inducible nitric oxide synthase by Src-family tyrosine kinases mediated through MyD88-independent signaling pathways of Toll-like receptor 4. Biochem. Pharmacol. 70, 1231–1240 (2005).
Kim, J. Y. et al. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem. Pharmacol. 79, 431–443 (2010).
Tu, S., Wu, W. J., Wang, J. & Cerione, R. A. Epidermal growth factor-dependent regulation of Cdc42 is mediated by the Src tyrosine kinase. J. Biol. Chem. 278, 49293–49300 (2003).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Curtiss, L. K. & Tobias, P. S. Emerging role of toll-like receptors in atherosclerosis. J. Lipid Res. 50 Suppl, S340–S345 (2008).
Lee, A. S. & Hendershot, L. M. ER stress and cancer. Cancer Biol. Ther. 5, 721–722 (2006).
Check, J. et al. Src kinase participates in LPS-induced activation of NADPH oxidase. Mol. Immunol. 47, 756–762 (2010).
Healy, S. J., Gorman, A. M., Mousavi-Shafaei, P., Gupta, S. & Samali, A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur. J. Pharmacol. 625, 234–246 (2009).
Cook, A. D., Braine, E. L. & Hamilton, J. A. The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J. Immunol. 171, 4816–4823 (2003).
Zinszner, H. et al. CHOP is implicated in programmed cell death inresponse to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Jefferson, L. S., Fabian, J. R. & Kimball, S. R. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int. J. Biochem. Cell Biol. 31, 191–200 (1999).
Tuckow, A. P., Vary, T. C., Kimball, S. R. & Jefferson, L. S. Ectopic expression of eIF2Bε in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E241–E248 (2010).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Feng, B. et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5, 781–792 (2003).
Kimball, S. R., Everson, W. V., Flaim, K. E. & Jefferson, L. S. Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am. J. Physiol. 256, C28–C34 (1989).
Rowlands, A. G., Montine, K. S., Henshaw, E. C. & Panniers, R. Physiological stresses inhibit guanine-nucleotide-exchange factor in Ehrlich cells. Eur. J. Biochem. 175, 93–99 (1988).
Matts, R. L. & London, I. M. The regulation of initiation of protein synthesis by phosphorylation of eIF- 2(α) and the role of reversing factor in the recycling of eIF-2. J. Biol. Chem. 259, 6708–6711 (1984).
Acknowledgements
This work was supported by postdoctoral fellowship grants from the Canadian Institutes of Health Research and the Heart & Stroke Foundation of Canada (C.W.W.); NIH-NHLBI grants HL75662 and HL57560 (I.T.); and NIH-NIDDK grants DK13499 and DK15658 (S.R.K.). We thank D. Ron (University of Cambridge, UK) for helpful discussions and for providing the CHOP–GFP reporter plasmid; C. Proud (University of Southampton, UK) for recombinant GST–DYRK2; D. Ren (University of Pennsylvania, USA) for advice regarding the use of mutant Src constructs; D. L. Brautigan (University of Virginia School of Medicine, USA) for the construct encoding PP2AcY307F; B. A. Hemmings (Friedrich Miescher Institute, Basel, Switzerland) for the construct encoding PP2AcL199P; and B. Berk (University of Rochester, USA) for an adenoviral vector encoding kinase-inactive Src.
Author information
Authors and Affiliations
Contributions
C.W.W. carried out the experiments and assisted with planning the experiments, data analysis and writing the manuscript; L.K. assisted with planning the experiments and data analysis; S.R.K. assisted with planning the experiments, data analysis and writing the manuscript; I.T. coordinated the project and assisted with planning the experiments, data analysis and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2179 kb)
Rights and permissions
About this article
Cite this article
Woo, C., Kutzler, L., Kimball, S. et al. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol 14, 192–200 (2012). https://doi.org/10.1038/ncb2408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2408
This article is cited by
-
Toll-like receptors 2 and 4 stress signaling and sodium-glucose cotransporter-2 in kidney disease
Molecular and Cellular Biochemistry (2023)
-
Impact of post-ruminally infused macronutrients on bovine mammary gland expression of genes involved in fatty acid synthesis, energy metabolism, and protein synthesis measured in RNA isolated from milk fat
Journal of Animal Science and Biotechnology (2020)
-
Mechanisms, regulation and functions of the unfolded protein response
Nature Reviews Molecular Cell Biology (2020)
-
Toll-Like Receptor 4 Activation Promotes Multiple Myeloma Cell Growth and Survival Via Suppression of The Endoplasmic Reticulum Stress Factor Chop
Scientific Reports (2019)
-
Causes and consequences of endoplasmic reticulum stress in rheumatic disease
Nature Reviews Rheumatology (2017)