Abstract
Testing for NRAS is now integral part in the assessment of metastatic melanoma patients because there is evidence that NRAS-mutated patients may be sensitive to MEK inhibitors, and RAS mutation is a common mechanism of acquired resistance during treatment with BRAF inhibitors. This study evaluated the sensitivity and specificity of immunohistochemical analysis using an N-Ras (Q61R) antibody to detect the presence of the NRASQ61R mutation in melanoma patients. A total of 98 primary cutaneous melanomas that have undergone examination of NRAS mutation were retrieved from a multicentric database. Formalin-fixed and paraffin-embedded melanoma tissues were analyzed for BRAF and NRAS mutations by independent, blinded observers using both conventional DNA molecular techniques and immunohistochemistry with the novel anti-human N-Ras (Q61R) monoclonal antibody (clone SP174). The antibody showed a sensitivity of 100% (14/14) and a specificity of 100% (83/83) for detecting the presence of an NRASQ61R mutation. Of the NRAS-mutated cases, none of the non-Q61R cases stained positive with the antibody (0/7). There were three cases with discordant NRAS mutational results. Additional molecular analysis confirmed the immunohistochemically obtained NRAS result in all cases, suggesting that a multiple analytical approach can be required to reach the correct sample classification. The reported immunohistochemical method is an accurate, rapid, and cost-effective method for detecting NRASQ61R mutation in melanoma patients, and represents a valuable supplement to traditional mutation testing. If validated in further studies, genetic testing would only be required for immunohistochemistry-negative patients to detect non-Q61R mutations.
Similar content being viewed by others
Main
Ras proteins are crucial crossroads of signaling pathways that link the activation of cell surface receptors with a wide variety of cellular processes leading to the control of proliferation, apoptosis, and differentiation.1 When acted upon by specific factors, such as extracellular ligands that bind specific membrane receptors, these proteins cycle between an activated and inactivated form, RAS-GTP and RAS-GDP, respectively.2 Activation requires dissociation of protein-bound GDP, a process that is accelerated by guanine nucleotide exchange factors. This switch-on process involves the reversible exchange of GDP for GTP. The switch-off process is entirely different and involves hydrolysis of GTP to GDP, the guanosine triphosphatase (GTPase) reaction, which is basically irreversible.
Mutations at positions 12, 13, or 61 of the HRAS, NRAS, and KRAS impair the GTPase activity of the carrier RAS proteins and lock them into a constitutively activated state in which they elicit downstream effectors, even in the absence of ligands that bind specific membrane receptors.3 Currently, mutations in NRAS, KRAS and HRAS are known to be present in 20%, 2%, and 1% of all melanomas, respectively. With regards to NRAS, the most common oncogenic change (80–90% of all NRAS mutations) reported is a point mutation at position 61, with mutations at positions 12 and 13 occurring less frequently.4
In the largest single-institution cohort of melanoma patients characterized for activating BRAF and NRAS mutations, the most common NRAS mutation was represented by the glutamine to arginine substitution at position 61 (NRASQ61R).5
NRAS has so far proven to be intractable to conventional drug discovery, and several different strategies of directly targeting RAS have not resulted in effective therapeutics. Nevertheless, there is evidence that some NRASQ61R-mutated cell lines are sensitive to MEK inhibition in vitro.6 Recently, an oral MEK inhibitor (MEK162) was tested in patients with metastatic melanoma harboring BRAF or NRAS mutations with encouraging results in NRAS-mutated patients, most of whom harbored NRASQ61R mutation.7 The response rate was reported in 20% of patients and progression-free survival was similar in BRAF- and NRAS-mutated patients. These data backed the hypothesis that RAS may be druggable in melanoma patients and several phase II and III studies are ongoing, most of them including NRAS-mutated metastatic melanoma (https://clinicaltrials.gov/).
In addition to the recent data on NRAS as a potential target, preclinical and clinical data suggest that: (1) in BRAF-mutated melanoma patients, a concomitant baseline mutation in the upstream NRAS oncogene may result in early lack of clinical benefit to BRAF inhibitors; (2) RAS mutation is a common mechanism of acquired resistance during treatment with BRAF inhibitors.8 Hence, testing for NRAS may be an integral part in the assessment of metastatic melanoma patients. Several molecular techniques have been developed to detect NRAS mutation such as conventional Sanger sequencing, pyrosequencing, or expensive and more sophisticated techniques including high-throughput multiplexed mass spectrometry-based platforms or targeted next-generation sequencing technologies.
However, for this purpose, tumor tissues may need to be sent to specialized laboratories for molecular testing, which inevitably results in a delay in defining the patient’s optimal management. Immunohistochemical analysis allows visualization of individual antigen-bearing tumor cells, is routinely performed and, in contrast to the molecular techniques, is more rapid, cheaper, and more accessible for clinical use.
With these considerations in mind, here we report, for the first time in the literature, the sensitivity and specificity of an anti-human N-Ras (Q61R) monoclonal antibody (clone SP174) in melanoma tissues. The encouraging results obtained in the current study on tissue samples from primary melanomas could be extended and validated on metastatic melanoma tissues as, in the era of personalized medicine, NRAS mutation status has become a key piece of information in the clinical management of melanoma patients. N-Ras (Q61R) immunohistochemistry could thus represent a screening tool in the assessment of NRAS mutant status in melanoma patients. In our view, N-Ras (Q61R) immunostaining seems to be complementary with mutational screening, as re-evaluation of discrepant cases may improve diagnostic accuracy.
Materials and methods
Immunohistochemistry on Melanoma Cell Lines
Human melanoma cell lines A375 (harboring wild-type NRAS) and SKMel-2 (harboring mutant NRASQ61R) were cultured in standard DMEM with 10% FBS. Paraffin embedding of cultured melanoma cells was performed under standard condition. Briefly, 107 cells per cell line were trypsinized, washed, and resuspended with 2 ml 4% PFA. Following gentle removal of the supernatant, the pellet was washed two times with PBS and gently resuspended with 600 μl Richard-Allan Scientific™ HistoGel™ Specimen Processing Gel (Thermo Scientific). The gelatinized gel blocks were then processed under standard condition for paraffin embedding and the section with 3 μm of thickness was stained with the rabbit monoclonal anti-human N-Ras (Q61R), clone SP174 antibody (Spring Biosciences, Pleasanton, CA, USA) (provided by UCS Diagnostic, Morlupo (Roma), Italy). The immunohistochemical staining was processed with automated staining system (Ventana Discovery; Roche).
Tumor Tissue Samples
A total of 98 patients (median age 73 years, range 16–99 years, 64 males, 34 females) with a diagnosis of primary cutaneous melanoma with Breslow thickness over 4 mm were identified from a retrospectively collected database. Tumor specimens were collected from the Pathological Anatomy Unit, Careggi University Hospital (Florence, Italy), and from the archives of Pathological Anatomy Units belonging to the Tumour Institute of Tuscany (ITT) network of Pathology. Twenty out of the 98 (20%) cases were from the head and neck region, 33 out of 98 (34%) from the trunk, 38 out of 98 (39%) from the extremities, and 7 out of 98 (7%) from acral sites. Hematoxylin- and eosin-stained sections were reviewed by two pathologists (DM and CU) to confirm the histopathological diagnosis. Representative sections from each lesion were selected for immunohistochemical analysis. From each tumor specimen, sections of formalin-fixed and paraffin-embedded samples were collected. The study was approved by the Institutional Ethical Committee and was performed according to the Declaration of Helsinki of 1975 as revised in 1983.
DNA Extraction
To obtain genomic DNA from formalin-fixed and paraffin-embedded blocks, 5-μm-thick tissue sections underwent proteinase K overnight digestion at 56 °C and extraction was performed using the formalin-fixed and paraffin-embedded Tissue Kit (Qiagen, Milan, Italy). Wild-type and mutated reference DNA were extracted from specific tumor cell lines: HeLa as wild-type control for BRAF and NRAS analysis, whereas SKMel-28 (homozygous for BRAFV600E) and HT1197 (heterozygous for NRASQ61R) were used as positive controls. DNA extraction from cell lines was performed using QIAamp DNA Mini Kit (Qiagen).
Direct Sequencing and High-Resolution Melting Analysis
DNA from formalin-fixed and paraffin-embedded samples were submitted to amplification by using 20 ng of total DNA in a PCR reaction mix of 25 ml. Samples were denatured for 9 min at 94 °C, followed by 40 cycles of amplification at 94 °C for 1 min, 58 °C for 1 min, and then 72 °C for 90 s, in a 2700 Thermal Cycler (Life Technologies, Monza, Italy). Total PCR products were purified using the Qiagen PCR Purification Kit (Qiagen). To perform the cycle-sequencing reaction, PCR products were blended with each primer (0.8 mol/l) in a Terminator Ready Reaction Mix containing Big Dye Terminators (Life Technologies) denatured for 1 min at 96 °C, and then submitted to 25 cycles at 96 °C for 10 s, 57 °C for 5 s, and 60 °C for 2 min. A second purification with the DyeEx 2.0 Spin Kit (Qiagen) was performed for big dye removal. A volume of 5 ml of purified cycle-sequencing product was then analyzed using an ABI PRISM 310 Genetic Analyzer (Life Technologies). For the HRMA melting profile, the analysis was performed as reported previously.9 Briefly, after PCR, samples were denatured with an initial hold of 5 min at 95 °C and 1 min at 40 °C and analyzed with a melting profile ranging from 75 to 85 °C for BRAF and 77 to 87 °C for NRAS in RotorGene 6000 (Corbett Research, Sydney, NSW, Australia). Sequences of primers used to perform analysis are reported in Table 1.
Sequenom Mass Spectrometry Genotyping
Discordant immunohistochemistry-negative and NRASQ61R mutation-positive results or discordant immunohistochemistry-positive and NRAS wild-type results were retested in DNA newly extracted from formalin-fixed and paraffin-embedded sections, at the Laboratory of Molecular Pathology, UO Pathological Anatomy III, Pisa, Italy, using a different molecular methodology, the Sequenom MassARRAY system (Sequenom), based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, together with the ‘Myriapod® Colon Status’ Kit (Diatech Pharmacogenetics, Jesi, Italy).
The ‘Myriapod® Colon Status’ Kit consists of a series of multiplexed assays designed to interrogate the hotspot mutations in four oncogenes: KRAS, BRAF, NRAS, and PIK3CA. Genomic DNA amplification and single base pair extension steps (iPLEX®) were conducted following the protocol provided by Diatech Pharmacogenetics. Briefly, the initial PCR amplification was performed in a 5 μl reaction mixture containing 20 ng of DNA under the following conditions: 95 °C for 2 min, 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 60 s for 45 cycles, and a final extension phase at 72 °C for 5 min. The remaining unincorporated dNTPs were dephosphorylates by adding 2 μl of a shrimp alkaline phosphatase (SAP) cocktail. The SAP reaction was then placed in a thermal cycler under the following conditions: 37 °C for 40 min, 85 °C for 5 min, and then held at 4 °C indefinitely. After PCR cleanup, a 2 μl of primer extension reaction cocktail (containing extension primer mixture, buffer, enzyme, and mass-modified ddNTPs) is added to the amplification products. Thermal cycling was performed under the following conditions: 94 °C for 30 s, followed by 40 cycles of 94 °C for 5 s, 5 cycles of 52 °C for 5 s, and 80 °C for 5 s, then at 72 °C for 3 min, and was finally held at 4 °C indefinitely. During the iPLEX® reaction, the primer is extended by one mass-modified nucleotide depending on the allele and the design of the assay. The primer extension reaction was then desalted by adding 41 μl of water and a cationic resin mixture, placed in a rotating shaker for 15 min and then centrifuged at 2000 r.p.m. for 15 min. Completed genotyping reactions were spotted in nanoliter volumes onto a matrix-arrayed silicon SpectroCHIP with 96 elements using the MassARRAY Nanodispenser (Sequenom). SpectroCHIP was analyzed using the Sequenom MassARRAY® Analyzer 4 spectrometer and the spectra were processed by the MassARRAY Typer Analyzer 4.0 software (Sequenom). All automated system mutation calls were confirmed by manual review of the spectra.
Immunohistochemistry on Human Melanoma Tissues
As a primary antibody, we used the rabbit monoclonal anti-human N-Ras (Q61R), clone SP174 (Spring Biosciences) (provided by UCS Diagnostic). The sections were dried at 60 °C for 30 min and stained with N-Ras antibody with a dilution of 1:80 on a Ventana BenchMark ULTRA immunostainer (Ventana Medical Systems, Tucson, AZ, USA). The Ventana staining procedure included pretreatment with cell conditioner 1 (pH 8) for 64 min, followed by incubation with antibody at 37 °C for 48 min. Antibody incubation was followed by standard signal amplification with the Ventana amplifier kit, UltraWash, and counterstaining with one drop of hematoxylin for 4 min and one drop of bluing reagent for 4 min. For chromogenic detection, ultraView Universal RED Detection Kit (Ventana Medical Systems) was used. Subsequently, slides were removed from the immunostainer, washed in water with a drop of dishwashing detergent, and mounted.
BRAF immunostaining was performed in one selected sample using the mouse monoclonal antibody anti-BRAF V600E, clone VE1 (Spring Bioscience, Pleasanton, CA) with a dilution of 1:30 (provided by UCS Diagnostics, Morlupo, Roma, Italy), according to the same protocol as described above. Anti-N-Ras (Q61R) and anti-BRAFV600E immunostaining was performed on the same tissue blocks used for mutational testing. No chromogen was detected when primary antibodies was omitted.
All immunostained slides were evaluated two times by two independent observers (DM and CS) blinded to all clinical, histopathological, and genetic data. The anti-human N-Ras (Q61R), clone SP174 antibody staining was scored as positive when the majority of viable tumor cells showed a distinctly clear cytoplasmic staining. More specifically, 60% of immunostained cells were requested to define a case as positive. The N-Ras antibody staining was scored as negative when there was no staining or weak staining of single interspersed cells. Isolated weak staining in single interspersed cells may be the result of an aspecific chromogen uptake of variably pigmented cells whose true nature (melanoma cells vs cells of histiocytic/macrophage lineage) can be difficult to assess with certainity on immunostained slides.
Discordant immunohistochemistry-negative and NRASQ61R mutation-positive results or discordant immunohistochemistry-positive and NRAS wild-type results were retested in DNA newly extracted from formalin-fixed and paraffin-embedded sections, at the Laboratory of Molecular Pathology, UO Pathological Anatomy III, Pisa, Italy, using a different molecular methodology (Sequenom mass spectrometry).
Statistical Analysis
Sensitivity, specificity, and positive and negative predictive values were calculated for the results of immunohistochemistry to predict for NRASQ61R mutation. The association between negative immunohistochemistry and mutation status was analyzed using the two-tailed Fisher’s exact test.
Results
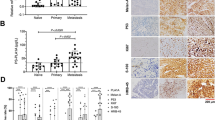
SKMel-2 melanoma cell lines carrying NRASQ61R mutation immunostained with the N-Ras (Q61R) antibody showed homogeneous and strong cytoplasmic staining, whereas the human melanoma cell line A375 harboring wild-type NRAS was negative (Figure 1).
Results of the mutational analysis on human melanoma tissues are reported in Table 2. One case was excluded because it could not be successfully amplified. All the detected mutations have been previously reported and are described in the Catalog of Somatic Mutations in Cancer (COSMIC; http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/).
Genotype characterization for BRAF exon 15 was obtained for 97/98 samples. Among them, 36/97 (37%) were mutated: 32 samples (33%) showed the p.V600E (c.1799T>A) variant, whereas 4 samples (4%) showed the p.V600K (c.1798_1799delinsAA) variant.
Mutations involving codon 61 of the NRAS gene were observed in 21/97 samples (22%). In particular, 14 samples (15%) showed the variant p.Q61R (c.182A>G), 3 samples (3%) the p.Q61K (c.181C>A) mutation, 2 samples (2%) the p.Q61L (c.182A>T), and 2 samples (2%) the p.Q61H mutation. Overall, NRASQ61R represented 67% of all RAS mutations.
Interestingly, a concomitant NRASQ61R and BRAFV600E mutation was found by Sanger DNA sequencing and immunohistochemistry, whereas a KRASQ61K mutation was detected in a BRAF and NRAS wild-type patient.
Of the 97 assessable cases, 20 cases were NRAS mutant on initial DNA sequencing (NRASQ61R=15, NRASQ61K=1, NRASQ61L=2, and NRASQ61H=2). Among these cases, two were immunohistochemistry-negative and NRASQ61R mutation-positive, whereas another case was immunohistochemistry-positive and NRASQ61R mutation-negative. These samples were retested on DNA extracted from subsequent sections of the same block that was used for initial sequencing and N-Ras (Q61R) staining. Two cases were confirmed as mutated also after retesting, notwithstanding that the two methods identified two different sequence variants (Q61K instead of Q61R). The other discordant case was resequenced and an NRASQ61R mutation was detected instead of the initial reported NRAS wild-type status (Table 3).
Of the NRAS mutant cases, none of the non-Q61R stained positively with the N-Ras (Q61R) antibody, and 14 of the NRASQ61R mutant tumors stained positively for the N-Ras (Q61R) antibody. Of the 75 NRAS wild-type melanomas, 75 were negative for the N-Ras (Q61R) antibody, and none stained positively (Table 4).
Using the mutational status from the retested discordant cases, the sensitivity of the N-Ras (Q61R) antibody was 100% (14/14; 95% confidence interval: 73–99%) and the specificity was 100% (83/83; 95% confidence interval: 94–99%). The positive and negative predictive values were 100% (14/14) and 100% (83/83), respectively (Table 5).
The two independent blinded observers were concordant in the assessment of N-Ras (Q61R) antibody staining in all cases; for one melanoma with high endogenous melanin content, concordance was achieved after re-evaluation at the double-headed microscope. N-Ras (Q61R) staining in melanoma cells appeared as diffusely and homogeneously moderate to strong cytoplasmic staining, with no background (Figures 2 and 3). The distribution of positivity among the positive melanoma cases was as follows: 60–80% of positive cells in 3/14 cases (21%) and 81–100% of positive cells in 11/14 (79%) cases. Intratumoral heterogeneity with N-Ras (Q61R) staining was observed only in one case (Figure 4), whereas in all other cases there was relatively uniform staining throughout the tumor. In one BRAFV600E- and NRASQ61R-mutated case, immunohistochemical staining with VE1 and N-Ras (Q61R) antibody performed on serial sections showed positivity for the two antibodies in same melanoma cell population (Figure 5).
NRAS wild-type melanoma negative with the N-Ras (Q61R) antibody (left); NRASQ61L-mutated melanoma negative with the N-Ras (Q61R) antibody (middle); and NRASQ61R-mutated melanoma staining positively with the N-Ras (Q61R) antibody (original magnification, × 40). Electropherograms (reverse strand) of sequencing analysis on NRAS exon 3 was performed in the same samples.
Intratumoral heterogeneity shown by N-Ras (Q61R) antibody (original magnification, × 2.5; square boxes original magnification, × 40). High-resolution melting analysis of NRAS exon 3: wild-type (blue) and heterozygous DNA (red) were used as reference for the analysis of the sample (green). Wild-type and mutated sequence from the same sample are also shown (reverse strand).
Immunohistochemical staining for VE1 and N-Ras (Q61R) on serial sections of a human melanoma harboring double mutations BRAFV600E and NRASQ61R (original magnification, × 5); High-resolution melting analysis and sequencing results (forward strand): data obtained from BRAF exon 15 (left) and NRAS exon 3 (right) are shown. Left column: hematoxylin and eosin stain—upper original magnification, × 5; lower original magnification, × 10).
Discussion
Testing for NRAS is now integral part in the assessment of metastatic melanoma patients for at least three reasons: (1) there is evidence that NRAS-mutated patients may be sensitive to MEK inhibitors; (2) in BRAF-mutated melanoma patients, a concomitant baseline mutation in the upstream NRAS oncogene may result in early lack of clinical benefit to BRAF inhibitors; (3) RAS mutation is a common mechanism of acquired resistance during treatment with BRAF inhibitors.
Furthermore, Johnson et al10 recently reported that patients with NRAS-mutated metastatic melanoma achieve increased clinical benefit from immunotherapy compared with those with BRAF/NRAS wild-type. These data suggest that NRAS mutation status may be a biomarker of response to immunotherapy in metastatic melanoma and that molecularly targeted immunotherapy may be feasible.
Among RAS mutations, NRASQ61R is the most common in the majority of studies.11, 12, 13, 14, 15 These results have been recently confirmed by a retrospective analysis in a large cohort of patients with advanced melanoma who underwent testing for both BRAF (exon 15) and NRAS (exons 1 and 2) mutations.5 Finally, it is important to underline that NRASQ61R has been reported to correlate with resistance to BRAF inhibitors in preclinical and clinical data.8, 16
Because testing for NRAS mutations currently requires the use of molecular techniques that are not available in most diagnostic pathology laboratories, testing may require a dispending laborious process before NRAS testing can commence. Furthermore, these molecular techniques are time consuming and expensive to perform and may require to be confirmed by different methodological approaches. On the contrary, mutation testing with immunohistochemistry offers many potential advantages: it is timely, inexpensive, widely available in all pathology departments, and requires minimal tissue.
This study compares an immunohistochemical-based method to identify the NRASQ61R mutant protein with PCR and sequencing-based mutation tests in melanoma patients. Our results suggest that the N-Ras (Q61R) antibody is highly sensitive (100%) and specific (100%) for the presence of the NRASQ61R mutation. To the best of our knowledge, this is the first time that these results are reported in the literature.
The current study included a consecutive cohort of 98 melanoma cases initially evaluated with direct sequencing and high-resolution melting analysis, and this allowed us detection of all relevant RAS mutations. Furthermore, discordant cases were subjected to Sequenom mass spectrometry genotyping, which permitted an accurate analysis of the positive and negative predictive values of the N-Ras (Q61R) antibody.
Initially there were three discordant cases, but after antibody staining and retesting either by sequencing or by Sequenom mass spectrometry, the correct molecular characterization could be obtained. Notwithstanding that the comparisons of different technologies for mutation detection is not the aim of the present paper, our data suggest that a multiple analytical approach may be required to reach the correct sample classification.
It has been reported that mutations in RAF and RAS are generally mutually exclusive. Several hypotheses have been advocated for this: RAS and RAF mutations appear functionally equivalent, or the RAS/RAF double mutation seems to be lethal for the cell. The immunohistochemical and molecular analyses revealed a concomitant RAS and RAF mutation in one patient from our series. The presence of both NRAS and BRAFV600E mutations in the same lesions is contrary to the current consensus that such mutations are almost always mutually exclusive in melanomas and other tumor types.17, 18, 19 However, Pollock et al20 observed concomitant NRAS and BRAFV600E mutations in 9% of nevi and suggested this might be because of different clonal nests of cells within these tumors carrying distinct mutations. In our case carrying both NRASQ61R and V600E mutations, immunohistochemistry with VE1 and N-Ras (Q61R) antibodies allowed direct visual confirmation of the BRAFV600E- and NRAS-mutated positive status in the same tumor cell population.
The consequences of concurrent mutations in BRAF and RAS in melanomas have been recently assessed only in terms of impact of these mutations on global gene expression. The definition of mutation-specific gene expression profiles indicate that the two mutations can signal not only through the common MAPK, but even by alternative signaling pathways, and may confer tumors with distinct biological features.21 It has been previously pointed out that although activating mutations coexist, they are mutually exclusive at the single-cell level.22
In the current study, intratumoral heterogeneity was seen only in one case, whereas intertumoral heterogeneity in the same patient (comparison of immunohistochemistry for NRAS mutational status of primary vs paired metastatic samples) was not an aim of the current study and future investigations will assess concordance of NRAS protein expression in multiple patient’s tumors. Previous immunohistochemical analyses using BRAFV600E antibody have shown that intratumoral heterogeneity of BRAF mutation is not generally observed23, 24, 25 and up to 100% concordance between matched primary and metastatic melanomas has been reported.24 These results support the routine use of immunohistochemistry with the anti-BRAFV600E antibody as an ancillary screening tool to assess the BRAFV600E mutation status in melanoma patients26 for treatment planning.
The strengths of this study are the following: a relative large series of patients evaluated both with immunohistochemistry and molecular analysis; the novelty of detecting the most frequently reported NRAS mutation in melanoma through immunohistochemical analysis; and the high sensitivity and specificity of the N-Ras (Q61R) antibody. Furthermore, in the first above-mentioned phase 2 prospective trials investigating MEK162 in NRAS-mutated melanoma patients, most of them actually harbored the NRASQ61R mutation.7
Nevertheless, we are aware that our study has limitations. This is indeed a retrospective series; as a consequence we cannot exclude a selection bias. Furthermore, as we evaluated through immunohistochemistry a relatively limited number of patients with NRAS mutation, our findings need to be confirmed in a prospective independent cohort. Furthermore, NRASQ61R does not recapitulate all NRAS-driven mechanisms of resistance to BRAF inhibitors, as NRASQ61K have also been reported to be associated with an acquired resistant phenotype.27
The focus of the current study was not on evaluating the frequency of NRASQ61R mutations in primary cutaneous melanoma, but on ascertaining the utility of a mutation-specific antibody. Therefore, our cases were not selected specifically to verify mutation rates. In any event, the frequency of BRAF and NRASQ61R mutations observed in the current study were 37% and 22%, respectively. The frequency of BRAF mutations was a little lower than expected28 and this may be owing to the fact that melanomas studied were not entirely on sites with intermittent sun exposure. The frequency of NRAS mutation in melanoma is 15–20% but if only melanomas confined to continuously sun-exposed sites are considered, the frequency rises up significantly.29
In conclusion, the N-Ras (Q61R) antibody is highly sensitive and specific for the detection of mutant NRASQ61R melanoma in formalin-fixed and paraffin-embedded samples. This immunohistochemical method has several advantages: it is cost effective, uses minimal tissue, and provides a result at the time of pathologic diagnosis. Use of the N-Ras (Q61R) antibody is a valuable supplement to traditional mutation testing, and if validated in further studies, genetic testing would only be required for immunohistochemistry-negative patients to detect non-Q61R mutations.
References
Malumbres M, Barbacid M . RAS oncogenes: the first 30 years. Nat Rev Cancer 2003;3:459–465.
Henis YI, Hancock JF, Prior IA . Ras acylation, compartmentalization and signaling nanoclusters [review]. Mol Membr Biol 2009;26:80–92.
Mor A, Philips MR . Compartmentalized Ras/MAPK signaling. Annu Rev Immunol 2006;24:771–800.
Mandala M, Merelli B, Massi D . NRAS in melanoma: targeting the undruggable target. Crit Rev Oncol Haematol 2006 doi:10.1016/j.critrevonc.2014.05.005(e-pub ahead of print).
Jakob JA, Bassett RL Jr, Ng CS et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012;118:4014–4023.
Solit DB, Garraway LA, Pratilas CA et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006;439:358–362.
Ascierto PA, Schadendorf D, Berking C et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249–256.
Nazarian R, Shi H, Wang Q et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973–977.
Mancini I, Pinzani P, Pupilli C et al. A high-resolution melting protocol for rapid and accurate differential diagnosis of thyroid nodules. J Mol Diagn 2012;14:501–509.
Johnson DB, Lovly CM, Flavin M et al. NRAS mutation: a potential biomarker of clinical response to immune-based therapies in metastatic melanoma (MM). J Clin Oncol 2013;31, (Suppl): (abstract 9019).
Colombino M, Capone M, Lissia A et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol 2012;30:2522–2529.
Fedorenko IV, Gibney GT, Smalley KS . NRAS mutant melanoma: biological behavior and future strategies for therapeutic management. Oncogene 2013;32:3009–3018.
Si L, Kong Y, Xu X et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer 2012;48:94–100.
Akslen LA, Puntervoll H, Bachmann IM et al. Mutation analysis of the EGFR-NRAS-BRAF pathway in melanomas from black Africans and other subgroups of cutaneous melanoma. Melanoma Res 2008;18:29–35.
Edlundh-Rose E, Egyházi S, Omholt K et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 2006;16:471–478.
McArthur G, Ribas A, Chapman PB et al. Molecular analyses from a phase I trial of vemurafenib to study mechanism of action (MOA) and resistance in repeated biopsies from BRAF mutation positive metastatic melanoma patients (pts) Proceedings of the American Society of Clinical Oncology, Chicago. J Clin Oncol 2011;29:3–7 (Suppl): (abstract 8502).
Davies H, Bignell GR, Cox C et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–954.
Omholt K, Platz A, Kanter L et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res 2003;9:6483–6488.
Thomas NE, Edmiston SN, Alexander A et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev 2007;16:991–997.
Pollock PM, Harper UL, Hansen KS et al. High frequency of BRAF mutations in nevi. Nat Genet 2003;33:19–20.
Downward J . PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol 2004;15:177–182.
Dadzie OE, Yang S, Emley A et al. RAS and RAF mutations in banal melanocytic aggregates contiguous with primary cutaneous melanoma: clues to melanomagenesis. Br J Dermatol 2009;160:368–375.
Long GV, Wilmott JS, Capper D et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol 2013;37:61–65.
Pearlstein MV, Zedek DC, Ollila DW et al. Validation of the VE1 immunostain for the BRAF V600E mutation in melanoma. J Cutan Pathol 2014;41:724–732.
Menzies AM, Lum T, Wilmott JS et al. Intrapatient homogeneity of BRAFV600E expression in melanoma. Am J Surg Pathol 2014;38:377–382.
Feller JK, Yang S, Mahalingam M . Immunohistochemistry with a mutation-specific monoclonal antibody as a screening tool for the BRAFV600E mutational status in primary cutaneous malignant melanoma. Mod Pathol 2013;26:414–420.
Trunzer K, Pavlick AC, Schuchter L et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 2013;31:1767–1774.
Decarlo K, Yang S, Emley A et al. Oncogenic BRAF-positive dysplastic nevi and the tumor suppressor IGFBP7—challenging the concept of dysplastic nevi as precursor lesions? Hum Pathol 2010;41:886–894.
van 't Veer LJ, Burgering BM, Versteeg R et al. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol 1989;9:3114–3116.
Acknowledgements
We are grateful to members of the Tumour Institute of Tuscany (ITT)-MelaPat Group who kindly contributed to the current study by providing tissue samples: Paola Apicella, Mauro Biancalani, Alessandra Calcinai, Augusto Giannini, Roberto Incensati, Stefania Innocenti, Barbara Loggini, Clelia Miracco, Lavinia Pugliese, Armando Rossi, Claudio Sabò, and Vanna Zucchi. This study was financially supported by Fondazione Cassa di Risparmio di Pistoia e Pescia (Pistoia, Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Massi, D., Simi, L., Sensi, E. et al. Immunohistochemistry is highly sensitive and specific for the detection of NRASQ61R mutation in melanoma. Mod Pathol 28, 487–497 (2015). https://doi.org/10.1038/modpathol.2014.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.137
This article is cited by
-
Detecting N-RAS Q61R Mutated Thyroid Neoplasias by Immunohistochemistry
Endocrine Pathology (2017)
-
NRASQ61R immunohistochemistry: a new tool for mutational status screening in challenging melanoma samples
Modern Pathology (2016)
-
BRAFV600E and NRASQ61R Homogeneity in Melanoma Tumors
Journal of Investigative Dermatology (2016)
-
NRAS Q61R , BRAF V600E immunohistochemistry: a concomitant tool for mutation screening in melanomas
Diagnostic Pathology (2015)
-
Cross-reactivity of the BRAF VE1 antibody with epitopes in axonemal dyneins leads to staining of cilia
Modern Pathology (2015)