Abstract
BRAF and NRAS are the most reported mutations associated to melanomagenesis. The lack of accurate diagnostic markers in response to therapeutic treatment in BRAF/NRAS-driven melanomagenesis is one of the main challenges in melanoma personalized therapy. In order to assess the diagnostic value of phosphatidylserine-specific phospholipase A1-alpha (PLA1A), a potent lysophospholipid mediating the production of lysophosphatidylserine, PLA1A mRNA and serum levels were compared in subjects with malignant melanoma (n = 18), primary melanoma (n = 13), and healthy subjects (n = 10). Additionally, the correlation between histopathological subtypes of BRAF/NRAS-mutated melanoma and PLA1A was analyzed. PLA1A expression was significantly increased during melanogenesis and positively correlated to disease severity and histopathological markers of metastatic melanoma. PLA1A mRNA and serum levels were significantly higher in patients with BRAF-mutated melanoma compared to the patients with NRAS-mutated melanoma. Notably, PLA1A can be used as a diagnostic marker for an efficient discrimination between naïve melanoma samples and advanced melanoma samples (sensitivity 91%, specificity 57%, and AUC 0.99), as well as BRAF-mutated melanoma samples (sensitivity 62%, specificity 61%, and AUC 0.75). Our findings suggest that PLA1A can be considered as a potential diagnostic marker for advanced and BRAF-mutated melanoma.
Similar content being viewed by others
Introduction
Although the incidence of malignant melanoma (MM) is less than 0.8% per 100,000 people, the mortality rate has significantly increased1,2,3. Indeed, over the past six decades, the overall mortality rate of MM has continuously increased by 6.5% per year in China4,5. Genetically, MM has one of the highest tumor mutation burden (TMB) across all solid tumors, with a mean mutation burden of over 20 mutations per megabase6. The mutations of the B-Raf proto-oncogene serine/threonine-kinase (BRAF), NRAS, and tumor protein p53 (TP53) are the main mutations that account for approximately 75% of all melanomas used for MM classification7,8,9. These mutations are associated with specific histopathological characteristics and melanomagenesis10,11,12. Melanoma patients with a defined TMB and carrying mutations in the cancer genome can benefit from early diagnosis13,14,15. An effective diagnostic strategy to identify BRAF/NRAS MM can allow the development of a subsequent targeted therapy that can improve the prognostic outcomes and therapeutic approaches among advanced MM patients11,16,17,18,19. The intra-tumoral heterogeneity of these genes leads to changes in the diagnostic and therapeutic strategies of melanoma, and personalized treatments and targeted therapies against melanoma have emerged15,20.
Our recent studies on multiple integrative and large-scale weighted gene co-expression network analysis identified phosphatidylserine-specific phospholipase A1-alpha (PS-PLA1A) as a novel network-based candidate marker in the diagnosis and prognosis of MM21. PS-PLA1 is a secreted cell membrane enzyme that is involved in catalyzing phosphatidylserine (PS) and 1-acyl-2-lysophosphatidylserine (lyso-PS) to hydrolyze fatty acids at the sn-1 position of these phospholipids. Recent studies showed that serum PLA1A levels are associated with tumor pathogenesis22,23,24, indicating its potential use as a diagnostic marker for monitoring several cancers including hepatocellular carcinoma25, gastric cancer26, colorectal cancer27, and melanoma28. PLA1A was identified as an individual marker in different genetically modified animals. PLA1A has been introduced PLA1A in laboratory medicine and in current clinical settings as an early-diagnostic metastatic and/or therapeutic marker27. mRNA and serum PLA1A levels in liquid biopsy diagnostics resulted promising tools for early diagnosis screening, tumor heterogeneity, and drug resistance of tumors with genomic mutations15,29.
Increasing mRNA and serum PLA1A levels are significantly associated with different clinical stages of melanoma, which suggests the potential involvement of the PS PLA1/lysophosphatidylserine axis in melanoma pathogenesis22,28. Despite numerous experimental studies, the diagnostic and prognostic values of PLA1A among different mutant melanoma patients and PLA1A role in melanomagenesis remain unknown. Certainly, effective diagnostic markers in the treatment of BRAF/NRAS-mutant melanoma are important in the development of a targeted therapy against advanced metastatic melanoma. Therefore, the aim of this study was at first to investigate the diagnostic value of PLA1A in melanoma patients at different stages and with different mutations to confirm the diagnostic value of PLA1A during melanomagenesis. Secondly, evidence in the use of PLA1A as a diagnostic and non-invasive therapeutic marker was documented to predict the clinical outcomes for advanced BRAF/NRAS-mutant MM.
Results
Clinicopathological findings
The demographic and clinicopathological features of the patients are summarized in Table 1. No differences between groups with regards to age and gender were found. A total of 41 patients were selected for this study according to the inclusion/exclusion criteria, who were then subdivided into three groups: 10 patients with naïve/normal melanoma (stage 0–I melanoma) diagnosed more than 2 years ago, 13 patients with primary melanoma (stage II–III melanoma) and 18 MM (stage III–IV melanoma). Among the 41 total patients, the genome of 22 (53%) females with a median age at diagnosis of 55 years was sequenced. Moreover, the melanomas were mostly isolated from the lower limb and hip (52%), as well as the trunk and neck (22%). The median thickness of melanoma was 2.2 mm in the primary and 5.4 mm in the metastatic group. Histologically, more than 54.8% (17/31) of melanoma patients were diagnosed through the pathological analysis of metastatic lymph nodes (67.8%) and metastasis in the lungs (19.4%). Furthermore, no patient was subjected to neoadjuvant therapy, and the 24% of melanomas patients underwent routine chemotherapy (Table 1).
PLA1A expression during melanogenesis
Previous screenings for PLA1 expression among different genders and stages of melanoma revealed that high serum PLA1A levels were associated with different clinical stages of melanoma in females28. Therefore, as a main member of PLA1 during melanogenesis, both PLA1A mRNA expression and serum levels were measured in our collected melanoma tissues and serum using quantitative Real-time PCR analysis (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA), respectively. PLA1A mRNA expression and serum levels significantly increased during melanogenesis (Fig. 1). Interestingly, the average PLA1A expression was consistently and significantly higher in tissues of metastatic melanoma (n = 15) compared to naïve/control melanoma samples (n = 10). In addition, PLA1A levels were significantly higher in primary melanoma tissues (n = 12) compared to naïve/control melanoma samples (Fig. 1A). Furthermore, serum PLA1A levels were significantly increased in metastatic (56.27 ± 21.23 µg/L) and primary (33.47 ± 13.59 µg/L) group compared to the naïve group (22.93 ± 11.53 µg/L) (p ≤ 0.001) (Fig. 1B). The invasive and aggressive indexes of PLA1A were compared by immunohistochemistry, with the invasive panel including Ki-67+, S-100+, P53+, HMB-45+, and Melena+ index evaluated in tumor sections (Fig. 1C). As expected, the expression of all the invasive indexes was significantly higher in patients with MM compared to that in the naïve/control melanoma patients (Fig. 1D). As illustrated in Fig. 1C, the percentage of PLA1A positive cells was 53.44 ± 23.34% in the metastatic group, 52.46 ± 32.67% in the primary group and 3.21 ± 1.67% in the naïve group. Similarly, HMB-45, S-100 and Ki-67 invasive indexes were highly expressed in more than 50% of the metastatic patients (p < 0.001). However, no significant differences were observed in PLA1A expression between primary melanoma tissue and metastatic tissue. Notably, PLA1A was highly expressed in advanced metastatic melanoma and invasive melanoma samples, as well as Ki-67, and they had the same HMB-45, S-100, and Ki-67 invasive index. Since PLA1A can be positive in more than 50 cases that are HMB-45, S-100 and Ki-67 positive, it can be a useful component in the diagnosis of metastasis melanomas by immunohistochemistry. Thus, these findings revealed that PLA1A suppressed melanoma tumor proliferation and angiogenesis in the metastasis of human melanoma cancer. Regarding the ratio of mRNA to serum PLA1A, the expression of PLA1A significantly increased during melanogenesis. Therefore, a significant relationship exists between PLA1A levels and melanoma stage.
Analysis of PLA1A expression among different patient samples. (A) The expression of PLA1A in normal naïve/normal melanoma (n = 10), primary melanoma (n = 13) and metastatic melanoma (n = 18) tissues measured using TaqMan MicroRNA assays kit. (B) Comparison of serum PLA1A levels (PS-PLA1A) between naïve/normal melanoma (n = 10), primary melanoma (n = 13) and metastasis melanoma (n = 18). Representative histological staining imaging (C) and quantification of the relative expression (D) of an invasive and aggressive index of PLA1A, in comparison to the invasive panel, Ki-67+, S-100+, P53+, HMB-45+, and Melena + index of tumor sections in various groups. *p < 0.05 and **p < 0.001 vs the naïve group; #p < 0.05 and ##p < 0.001 vs the primary group.
Correlation of PLA1A expression with melanogenesis
The diagnosis of MM represents a challenge among a wide range of melanoma samples with different morphologies and immunohistochemical features. In order to verify whether PLA1A is associated with a type of diagnostic marker of MM, the linear logistic regression analysis was performed to predict the accuracy of the detection of PLA1A expression and characteristics of MM (Fig. 2). Figure 2A depicts the correlation of PLA1A with common diagnostic markers of MM. The relative PLA1A levels are positively correlated to S-100 and HMB-45 (r = 0.293 and r = 0.353 respectively, both p ≤ 0.001; Fig. 2B,C). By simultaneously considering both tissue and serum levels of PLA1A, the results revealed that PLA1A was inversely correlated to Melan-A (r = − 0.025, Supplementary Fig. 1A). Strikingly, PLA1A was not correlated to the expression of Ki67 (r = 0.033, p = 0.133; Supplementary Fig. 1B) and TP-53 (r = 0.01, p = 0.245; Supplementary Fig. 1V). In general, higher PLA1A levels were correlated with disease severity and metastatic lesions among patients with melanoma.
Correlation coefficient of PLA1A with diagnostic markers across metastatic melanoma. (A) Linear logistic regression analysis to predict the accuracy of PLA1A expression in comparison to the routine diagnostic markers of malignant melanoma. By considering both mRNA and serum levels, PLA1A was found as correlated to the expression of S-100 (B), HMB-45 (B), BRAF (C), and NRAS (E) in naïve/normal melanoma (green cycle), primary melanoma (blue cycle) and metastasis melanoma (red cycle).
Previous studies demonstrated that patients with BRAF/NRAS-mutant exhibit enhanced invasive characteristics, which are correlated to disease severity10,14. Therefore, in order to evaluate the value of PLA1A expression as a diagnostic marker in the treatment of BRAF/NRAS-mutant melanoma, the expression of PLA1A in BRAF and NRAS mutant samples was evaluated through the use of the linear logistic regression in Fig. 2D,E, respectively. The results showed that PLA1A was positively correlated to the frequency of BRAF and NRAS (r = 0.136, p = 0.01; r = 0.065, p = 0.059, respectively). In total, the positive correlation of PlA1A with BRAF and NRAS led to the hypothesis that PLA1A might be a potential diagnostic marker in BRAF/NRAS-driven melanogenesis.
PLA1A expression as a prognostic marker in BRAF/NRAS-mutated samples of melanoma
It is clear that a specific combination between BRAF and NRAS mutations leading to up- or downregulation of novel diagnostic marker in MM may be more consistent with the diagnosis of advanced stage III melanoma30,31. Therefore, the diagnostic value of PLA1A in BRAF and NRAS-mutated samples of melanoma patients was investigated to confirm the diagnostic utility of PLA1A during melanomagenesis. The BRAFV600E and NRASP29S mutation status of each tumor was determined using Sanger sequencing as previously described (Fig. 3A)32,33,34. Overall, 54% of the tumors in the discovery cohort had canonical BRAFV600E mutation, and 46% were NRASP29S (Fig. 3B). Interestingly, the proportion of BRAFV600E (12 of 22) and NRAS-mutants (10 of 19) was higher in the metastatic group compared to the one in the naïve/control group (p ≤ 0.001) (Table 2). These findings confirmed that high BRAF/NRAS-mutated tumors were associated with a more severe stage of melanoma. A significant relationship was found between advanced melanoma stages and increased BRAF/NRAS mutation rate (p ≤ 0.001).
BRAF- and NRAS-mutant melanoma rates among different study groups. (A) Summary of the genetic landscape of BRAF V600E and NRAS P29S mutant genes in normal naïve/normal melanoma (n = 10), primary melanoma (n = 13) and metastasis melanoma (n = 18). (B) Bars represent the percentage of somatic BRAF V600E and NRAS P29S mutations, distinguished by their individual colors.
PLA1A expression in BRAF-WT/MUT type and NRAS-WT/MUT type by immunohistochemistry is shown in Fig. 4A. Morphometrically, all high-grade nodular MM samples, which were categorized as BRAF/NRAS-MUT, showed high PLA1A expression with significant intensity. Additionally, PLA1A expression was enclosed in the well-circumscribed foci of BRAF+ samples in both metastatic and primary samples, which were analyzed over the whole sections. In contrast, PLA1A was highly distributed over the entire tumor section and not just in the focal positive section. In addition, a significant difference in the increased PLA1A positive cells was found between advanced BRAF/NRAS-MUT melanoma stages and naïve/control melanoma samples. By considering both PLA1A mRNA and serum levels simultaneously, PLA1A levels were significantly increased in BRAF/NRAS+ melanoma patients (p ≤ 0.05; Fig. 4B,C). The ratio of PLA1A was significantly higher in patients possessing the BRAF-MUT (p = 0.019 for PLA1A mRNA and p = 0.004 for serum PLA1A levels; Fig. 4B) compared to the BRAF-WT patients. Similar to the results from BRAF+ melanoma patients, serum PLA1A levels were increased in MUT-NRAS melanoma patients compared to the level in the WT-NRAS (36.25 ± 16.24 µg/L vs 29.56 ± 14.26 µg/L), although this difference was not statistically significant (Fig. 4C; p > 0.05). These results suggested that enhanced PLA1A mRNA and serum levels are significantly present in BRAF+ melanoma patients, compared to NRAS+ melanoma patients. Interestingly, our results indicated that both BRAF+ and NRAS+ MM patients had high PLA1A expression compared to the expression in primary and naïve/control patients (Supplementary Fig. 2). In general, these findings suggested that PLA1A could be considered as a potential diagnostic marker in BRAF-driven melanomagenesis.
PLA1A expression in BRAF- and NRAS-mutant subjects with melanoma. (A) Gallery of representative immunostaining of PLA1A expression in BRAF-WT/MUT type (orange) and NRAS-WT/MUT among naïve, primary, and metastatic groups. Patents with wild and mutant type are represented by the blue and orange box, respectively. (B) The histogram shows the frequency of PLA1A mRNA expression and serum levels between BRAF-WT (green) and BRAF-MUT (Red) type of patients. (C) Distribution of PLA1A expression in patients with NRAS-WT (green) and NRAS-MUT (Red) type.
Diagnostic value of PLA1A in advanced MM
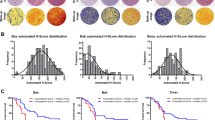
Finally, our patients were categorized into groups according to their PLA1A levels as lower than, equal to, or higher than the median expression of PLA1A (FC: 0.072 for PLA1A mRNA expression and 40.91 µg/L for serum PLA1A levels) to determine any possible relationship between PLA1A expression and disease-free survival (DFS) of melanoma patients using the Kaplan–Meier method and log-rank test (Fig. 5A). By considering both PLA1A mRNA and serum levels, 56% (23 of 41 patients) of the patients were categorized as either low-PLA1A (FC > 0.072 for PLA1A mRNA expression and > 40.91 µg/L for serum PLA1A levels), while almost 44% (18 of 41 patients) of the patients were categorized as a high-PLA1A (FC ≥ 0.072 for PLA1A mRNA expression and ≥ 40.91 µg/L for serum PLA1A levels). As expected, melanoma patients with PLA1A1-high tumors had significantly shorter DFS compared to that in patients with PLA1A-low tumors (5-year DFS rate: 69.3% vs 75.4%, p = 0.026) (Fig. 5A). This result indicated that the prognosis of patients with high-PLA1A tumors was worse compared to that of patients with low-PLA1A tumors (p < 0.05, Fig. 5A). The correlation between different stages of melanoma and 5-year DFS is shown in Fig. 5B. The area under the curve (AUC) of the receiver operating characteristic (ROC) analysis of naïve/primary, naïve/metastasis, and primary/metastasis group was 0.99, 0.99, and 0.67, respectively. The analysis based on specimen types indicated that PLA1A had a relatively accurate diagnostic value in discriminating the naïve melanoma samples from advanced melanoma samples, with a sensitivity of 0.91 and specificity of 0.57 (Fig. 5B and Supplementary Fig. 3). By considering both PLA1A mRNA and serum levels, the univariate analysis among BRAF/NRAS mutated samples demonstrated that PLA1A was more accurate for BRAF-MUT samples compared to NRAS-MUT samples (Fig. 5C). As shown in Fig. 5C and Supplementary Fig. 3B, the pooled sensitivity and specificity were higher in the BRAF-MUT samples compared to NRAS-MUT samples (Table 2). Moreover, PLA1A had the highest sensitivity, specificity, and AUC in BRAF-MUT advanced melanoma samples, suggesting that PLA1A could be used as a marker for an effective prediction and diagnosis of advanced BRAF-mutant melanoma cancer.
Diagnostic value of PLA1A in advanced metastasis melanoma. (A) The disease-free survival (DFS) rate for PLA1A expression in 23 patients with low-PLA1A and 18 patients with high-PLA1A. The patients were categorized as either low-PLA1A when FC > 0.072 for PLA1A mRNA expression and > 40.91 µg/L for serum PLA1A levels. The high-PLA1A is FC ≥ 0.072 for PLA1A mRNA expression and ≥ 40.91 µg/L for serum PLA1A levels. (B) Receiver operating characteristic (ROC) curve for PLA1A expression between normal naïve/metastasis melanoma group (red line), naïve/primary melanoma (green line), and primary/metastasis melanoma (blue line). (C) ROC curve to determine the optimal cut‐off value of PLA1A to distinguish melanoma patients with BRAF-mutation (blue line) and NRAS-mutation (orange line). The diagnostic values were calculated by considering both PLA1A mRNA expression and serum levels.
Discussion
Our results revealed for the first time that the upregulation of PLA1A in the serum of advanced metastatic melanoma tissues represent an excellent diagnostic marker in BRAF/NRAS-driven melanomagenesis. Besides, the upregulation of PLA1A mRNA in tissue samples of advanced MM patients represents a more accurate diagnostic marker. A significant positive correlation was found between PLA1A and disease severity, as well as routine diagnostic markers such as HMB-45, S-100 and Ki-67, which supports the idea that PLA1A expression could be correlated to diagnostic markers in MM, and is involved in melanogenesis among advanced melanoma human subjects. Therefore, during the melanogenesis, the serum PLA1A level could be used as a non-invasive marker in the diagnosis of MM. The evidence to consider PLA1A as a diagnostic marker were shown, which could help to distinguish naïve melanoma samples from advanced melanoma samples. These findings suggested that PLA1A could be considered as a clinical marker for an accurate prediction and diagnosis of advanced BRAF-mutant melanoma cancer.
Reliable diagnostic markers and the assessment of the molecular status of the tumor in the patients are required for an effective targeted therapy in the treatment of high TMB melanoma, leading to an approved adjuvant therapy that can be used among patients with advanced MM11,12,20. To the best of our best knowledge, we are the first showing that current serological and immunohistochemical markers in melanoma had significantly limited the detection of the advanced stage of this disease, which metastasized from the primary site35,36,37. Among solid advanced melanoma tumors, it is difficult to find the markers precisely classifying the stage of melanoma, resulting in the currently known sensitivity and resistance to therapeutic agents of specific mutated patients. Furthermore, most of the serological and histological diagnostic markers of melanoma are based on the detection of melanocytes, rather than melanoma itself37. Likewise, BRAF/NRAS mutant melanoma tumors contain several high-frequency driver mutations, and therefore represent a big challenge to dermatologists or oncologists in the discovery of unique and stable upregulated oncogenic markers among highly invasive melanoma samples for an early diagnosis and targeted therapy19,38,39. Therefore, novel markers are mostly based on genomic markers of carcinogenetic gene mutations40. Accordingly, MM patients that are positive for BRAF/NRAS mutation increased their survival with treatments involving novel therapeutic markers39,40.
The serum PLA1A level is associated not only with tumorigenesis, but also to most inflammatory diseases, including systemic lupus erythematosus, hepatitis, and hyperthyroidism41,42,43. Extensive evidence demonstrated that PLA1A regulates different stages of carcinogenesis associated with angiogenesis, differentiation, proliferation, invasion, apoptosis, and metastasis44. PS-PLA1, the sole substrate of LysoPS, is detectable among several physiological conditions and is normally restricted to the inner surface of the cell membrane, apoptotic cells, antigen-activated lymphocytes and immunological escape of melanoma cells45. It is clear that the cause of MM is mostly related to mutagenesis, with the involvement of several oncogenes, including BRAF and NRAS40. Thus, in this work, PLA1A was evaluated for the first time among the histopathological subtypes of the BRAF/NRAS-driven melanoma cancer population. As no therapeutic agents have been approved specifically for NRAS-mutant melanoma, our aim was to compare the benefit of PLA1A in the BRAF- and NRAS-driven melanoma cancer population39,46. Our results showed that high serum PLA1A levels were associated with tumors invasion, as well as a higher incidence of BRAFV600E and NRASP29S mutation metastasis. Interestingly, V600E hotspot mutations were significantly overexpressed among subcutaneous metastases. Thus, it is possible that V600E hotspot mutation on BRAF leads to more than one NRASP29S mutation, which can induce different physiological functions in response to LysoPS, leading to increased serum PS-PLA1 levels in melanoma patients. Furthermore, the expression of LysoPS receptors differs among BRAF-mutant melanoma samples, as well as in other broad perspective of TMB-encompassing cells, immunomodulatory cells, and other cell types47. Prospectively, further functional studies on the effect of PS-PLA1A/LysoPS receptor axis on melanogenesis could additionally confirm PLA1A as a potential therapeutic target for different types of TMB-driven melanomagenesis.
In addition, S-100 and HMB-45 are excellent immunohistochemical markers with a sensitivity and specificity of 100% in distinguishing melanoma from non-melanocytic carcinomas48,49. In this regard, our present work demonstrated that the relative PLA1A levels were positively correlated to both S-100 and HMB-45 markers. Because of the poor 5-year survival rate and prognosis of patients with late stage melanoma, PLA1A, in conjunction with S-100 and HMB-45, could serve as a novel marker to distinguish lymph node naive melanoma from melanoma metastasis49. However, a further clinical confirmation is needed to determine the diagnostic and prognostic value of PLA1A levels in melanoma, as well as to establish which patients need more aggressive therapy50.
As regard the prototypic testing of advanced cutaneous melanoma, PLA1A increased the hope in finding effective clinical markers for high TMB, and a potential cure15,29. Interestingly, this is the first report demonstrating that PLA1A mRNA expression, with a cut-off value of 0.072 and serum PLA1A levels of 40.91 µg/L could be used to discriminate naïve melanoma samples from advanced melanoma samples, with a sensitivity of 91% and a specificity of 57%. Previous studies proposed that the relatively low specificity of PS-PLA1 may be due to the increased serum PS-PLA1 levels in control patients, although these are still lower compared to those observed in patients with primary melanoma28,42. Importantly, the high sensitivity, specificity, and AUC in BRAF-MUT advanced melanoma samples suggested that PLA1A could be used as a marker for an effective prediction of advanced BRAF-mutant melanoma cancer. Therefore, the overall diagnostic utility of the measurement of PLA1A mRNA expression or serum levels in BRAF-mutant MM was unsatisfactory. Whether or not it is combined with other markers, the diagnostic value of PLA1A as an innovative therapeutic target combined with BRAF inhibition requires further elucidation.
Our finding showed for the first time that PLA1A with the highest sensitivity, specificity, and AUC could be used as a single indispensable marker for diagnosis, screening, and prognosis in BRAF-MUT advanced melanoma. Similarly, our results proposed to combine PLA1A with other routine invasiveness diagnostic markers of melanoma: HMB-45, S-100 and Ki-67, with the hope that these markers, after proper standardization, might serve as a potent indicators in the prognosis and determination of the metastatic stage of melanoma51. In precision oncology, real-time molecular monitoring of diagnostic markers is used to identify patients with a different stage, but it should also play a critical role in finding markers that predict the treatment outcome in patients with different molecular classification and specific subtype52. In this regards, single-site biopsy of PLA1A could be considered as a diagnostic marker of BRAF-mutant metastasis in melanoma cancer, since it is sufficiently sensitive and precise in distinguishing melanoma patients with BRAF-mutated and NRAS-mutated advanced melanoma. Thus, investigating multiple mRNA marker profiles would be beneficial to improve the sensitivity of PLA1A as a single diagnostic marker or as a novel component in the immunohistochemical panel of metastatic melanomas. Surely, this experimental research should be followed by a further work to characterize the clinical applicability of PLA1A, which is actually underway.
These results from this study clearly adhere to the idea that PLA1A is a potential diagnostic and significant therapeutic marker among advanced MM. These results point to the probability that PLA1A imbalance and clarification of its characteristics can help to recognize the pathogenesis of melanoma, which may be partially due to the serum and lung tissue microenvironment. A small sample size, less functional studies, and less homogeneous distribution of samples based on phytology and mutation parameters might be a springboard for large sample size with well-pathologically characterized melanoma samples in future studies. The clinical use of PLA1A, a novel therapeutic target of MM progression, requires further studies that are currently ongoing in our laboratory.
In conclusion, our results showed that PLA1A might be a promising diagnostic marker in patients with advanced metastatic melanoma, providing a more accurate diagnostic marker for BRAF-mutant samples of MM patients. A significant positive correlation was found between PLA1A and disease severity as well as diagnostic markers, thus PLA1A could be used in the future as a non-invasive marker in the diagnosis and therapy of metastatic melanomas. Further well-designed cohort studies are needed to establish the clinical significance of PLA1A for personalized MM diagnosis.
Methods
Ethic statement
This study was approved by the “Ethics Review Board” at the Affiliated Hospital of Southwest Medical University (No. KY2019041). Written informed consent in agreement to the requirement of the Declaration of Helsinki (1983 Revision) was obtained from all participants or their guardians prior to the study to use their clinical and pathology information, as well as for mutation analysis. The volunteers were informed about the goal and protocols of the study. Additionally, all clinical assessments were performed according to the local Ethics Committee guidelines of the Pathology Department and Oncology Department at The Affiliated Hospital of Southwest Medical University in Luzhou, Sichuan, China.
Patient population and clinical assessment
Patients with melanoma that were preliminarily selected for this prospective study were confirmed as having the disease by two expert oncologists (S.I. and Q.L.W.), as well as one pathologist (C.Z.). All participants were adult melanoma patients who were referred to the Department of Dermatology and Oncology of the Affiliated Hospital of Southwest Medical University, Luzhou, China from April 2020 to September 2020. Melanoma was diagnosed according to the World Health Organization (WHO) guidelines and the pTNM Union for International Cancer Control (UICC) pathological staging criteria. All patients were treated at the Department of Dermatology and Oncology, the Affiliated Hospital of Southwest Medical University, Luzhou, China. The samples were collected from naïve/normal melanoma subjects with stage 0–I and primary melanoma subjects with stage II–III before the treatment, or subjects with stage III–IV melanoma when they did not receive any treatment except palliative care. The demographic and histopathologic variables of all subjects, including medical, reproductive and family history, tumor site, histological type, treatment and survival were correctly recorded in a database. Metastasis was assessed by fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging. All metastatic organs were evaluated and confirmed by two expert pathologists (C.Z. and Q.L.W.). In addition, laboratory tests including blood routine, and liver function tests were performed in patients who need a confirmation for the presence of metastases. All patients with MM were clinically stable and had not experienced any malignancy for ≥ 2 months prior to the inclusion in the study. Participants with some autoimmune disorders and systemic inflammatory disorders such as type 1 diabetes mellitus, interstitial lung disease, rheumatoid arthritis, or other immune related diseases were excluded. In addition, history of acute bronchiolitis/pneumonia, systemic lupus erythematosus, thyroid disorders, multiple metastatic sites, and/or participation in simultaneous clinical trials were considered as exclusion criteria. Disease stages were classified according to the AJCC 8th edition53. All patients underwent standard treatments based on the practice of each treating physician. The demographic and baseline clinico-philological characteristics of patients are listed in Table 1.
Serum samples
The serum samples used in this study were residuals samples that were obtained from all subjects when they did not undergo any treatment, with the exception of palliative care. To obtain the serum samples, 2 mL blood were left to clot at room temperature for at least 30 min, and then centrifuged at 1200×g for 8 min. The serum was collected and subsequently divided into 500 µL aliquots, which were then stored at − 80 °C until further DNA and RNA extraction.
Mutation analysis
In order to detect V600E mutation in BRAF gene (c.1796T > A) and P29S in NRAS gene (c.85C > T), DNA was isolated from 5 μm-thick sections of all tissue samples through the use of QIAamp DNA FFPE Tissue kids, according to the manufacturer’s protocol. These recurrent mutations in BRAF and NRAS genes were the most common genetic alterations in melanoma, and were identified via qRT-PCR through the use of specific hybridization probes that allow the detection of the following variants9,13,14. Primer sequences and specific PCR reactions are available on Supplementary Table 1. The concentration of extracted DNA was measured using a NanoDrop2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The optical density ratio 260/280 ~ 1.8 and 260/230 > 1.5 was assessed to evaluate the quality. The detection of variants was performed using LightCycler 96 real-time PCR machine, according to standard protocols as previously described54,55. PCR products were confirmed via Sanger sequencing methods on an ABI-3500 sequencer (Applied Biosystems Inc., Foster City, CA, USA). Finally, the resulting sequences were compared to consensus sequences via Seqman software (Lasergene 8.0; DNASTAR, Inc., Madison, WI). All reactions were performed with two replicates per sample, as well as a non-reverse transcription control and non-template control for each test.
Quantitative real-time PCR analysis
In order to quantify and compare the expression of PLA1A1 gene with the NRAS and BRAF genes, qRT-PCR was performed based on the standard protocols as previously described56. In brief, total RNA was extracted from frozen biopsies using Trizol reagent (Takara, Dalian, China), according to the manufacturer's instructions. The expression of target genes was quantified using predeveloped TaqMan assays from Applied Biosystems (Life Technologies, Foster City, CA) and the expression was normalized to that of the 18SRNA housekeeping gene using the comparative Ct method57,58. Primer sequences used in this investigation are listed in Supplementary Table 1.
ELISA measurements
Serum levels of human PLA1A were measured using the standard specific double-antibody sandwich ELISA kits, according to the manufacturer’s instructions among three study groups (1:1000 dilution; ABIN5072368, Biotech Co. Technology, Beijing, China)26,27. The lower limit of detection of each kit (supplier's data) was 18.75 pg/mL. The ELISA reader and washer were Stat-Fax 2100 and Stat-Fax 2600 (Awareness Technologies, FL, USA), respectively.
Immunohistochemistry
The best frozen sample was used for hematoxylin and eosin analysis, as well as histological conformation. Immunostaining of PLA1A, S-100, HMB-45, Melan-A, Ki-67, and P53 was performed using the streptavidin–biotin alkaline phosphatase complex method by the Vectastain ABC-AP standard kit (Vector Laboratories; Burlingame, CA) as previously described59,60. In brief, antigen retrieval was achieved by heating the 5 μm-thick paraffin-embedded sections in a high-temperature pressure cooker for 90 s in citrate buffer at pH 6.0. Then, endogenous peroxidase activity was suppressed by the treatment with methanol + 1% hydrogen peroxide for 30 min at room temperature. Nonspecific reactions and quench endogenous alkaline phosphatase were blocked using 2% normal mouse serum for 30 min at RT (Santa Cruz Biotechnology, Santa Cruz, CA) and levamisole (Vector Laboratories, Burlingame, CA), respectively. The slides were incubated using the primary monoclonal antibodies of the proteins mentioned above, based on the related concentration (see Supplementary Table 2). The endogenous peroxidase activity was quenched in 0.5% H2O2 for 10 min, and the slides were incubated with primary monoclonal antibody, according to the manufacturer's guidelines. Subsequently, the slides were incubated in a humid chamber for 10 min at RT with biotinylated secondary antibody, according to the manufacturer’s instructions (Vector Laboratories; Burlingame, CA). Then, an avidin–biotin alkaline phosphatase complex was added for 30 min at RT (3 µl 1:400 Promega, Madison, WI), and the color was developed using the Vector Red alkaline phosphatase substrate kit (Vector Laboratories). Additionally, normal mouse IgG, normal preimmune rabbit IgG, or Tris-buffered saline was used as negative control. The patients were coded, and measurements were made in a blinded mode by two expert pathologists (S.Y. and C.Z.). The Ki-67, P53 and S-100 labeling index were calculated on five randomly selected fields of each tumor sample as number of the invasive index positive cells/total counted cells at 400× magnification. In addition, five high power visual fields (400×) were randomly selected for each section to calculate the percentage of area with a positive expression of HMB-45 and Melan-A. The proportion of HMB-45 and Melan-A positive expression was calculated as area of HMB-45 and Melan-A positive staining/the total area under a high magnification field of vision. Since PLA1A staining is mainly observed in the cytoplasm of cancer cells, the immunostaining intensity of PLA1A was classified into three grades: negative-to-weak, moderate, and strong. Sections where more than 30% of cells were scored as negative-to-weak were classified as PLA1A-negative, and those with 30% or less were moderately classified as PLA1A-low, and 40% of all samples that had strong expression of PLA1A immunostaining were classified as PLA1A-high. All these counts were performed in a blinded manner. All sections were visualized using a Zeiss Axioplan 2 microscope, and images were captured on a Windows NT workstation and analyzed using Zeiss Axiovision software (Zeiss; New York, NY).
Statistical analysis
Statistical analysis was performed using SPSS software version 21.0 (Chicago, Illinois, USA). All tests were repeated three times or more. Results were presented as mean ± Standard Deviation (SD) or median (range). Serum PLA1A levels were compared among groups using the one-way Kruskal–Wallis test. If the results of this test indicated significance, a Mann–Whitney test was used for post-hoc analysis to compare two groups, with corrections of the P-values conducted according to Bonferroni. A Spearman’s rank correlation coefficient test was employed to determine the association between two variables. Linear stepwise multivariate regression was performed to identify parameters that correlated with serum PLA1A levels. Furthermore, DFS was analyzed using the Kaplan–Meier method, log-rank test, and Cox proportional hazard model. In order to identify the cut-off threshold effects and construct the ROC curves, spearman’s rank correlation coefficient test was used to determine the relationship between two sensitivity (ordinate) and one specificity (abscissa)61. The results were considered statistically heterogeneous when p < 0.05 and/or I2 > 50%62. The diagnostic threshold effect was analyzed using the Spearman correlation coefficient test. In all tests, two-sided p values less than 0.05 were considered statistically significant. All graphs were designed using GraphPad Prism software 5.0 for windows (GraphPad Software Inc., La Jolla, CA, USA).
Data availability
All data associated with this study are present in the paper or the Supplementary Information. Microarray datasets were submitted to Gene Expression Omnibus (GEO) public repository and data can be accessed through accession number GSE34460, GSE18509, GSE24996, GSE7553, GSE15605, and GSE19234. Source data are provided in this article21.
References
Tas, F. Metastatic behavior in melanoma: Timing, pattern, survival, and influencing factors. J. Oncol. 2012, 647684 (2012).
Francken, A. B. & Hoekstra, H. J. Follow-up of melanoma patients: The need for evidence-based protocols. Ann. Surg. Oncol. 16, 804–805 (2009).
Jemal, A. et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J. Am. Acad. Dermatol. 65(S17–25), e11-13 (2011).
Wu, Y. et al. Burden of melanoma in China, 1990–2017: Findings from the 2017 global burden of disease study. Int. J. Cancer 147, 692–701 (2020).
Zhang, M. & Zhang, N. Clinical and prognostic factors in 98 patients with malignant melanoma in China. J. Int. Med. Res. 45, 1369–1377 (2017).
Davar, D., Lin, Y. & Kirkwood, J. M. Unfolding the mutational landscape of human melanoma. J. Investig. Dermatol. 135, 659–662 (2015).
Reddy, B. Y., Miller, D. M. & Tsao, H. Somatic driver mutations in melanoma. Cancer 123, 2104–2117 (2017).
Davis, E. J., Johnson, D. B., Sosman, J. A. & Chandra, S. Melanoma: What do all the mutations mean?. Cancer 124, 3490–3499 (2018).
Forbes, S. A. et al. COSMIC: Mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945-950 (2011).
Ponti, G. et al. BRAF, NRAS and C-KIT advanced melanoma: Clinico-pathological features, targeted-therapy strategies and survival. Anticancer Res. 37, 7043–7048 (2017).
Jenkins, R. W. & Sullivan, R. J. NRAS mutant melanoma: An overview for the clinician for melanoma management. Melanoma Manag. 3, 47–59 (2016).
Lyu, J. et al. Mutation scanning of BRAF, NRAS, KIT, and GNAQ/GNA11 in oral mucosal melanoma: A study of 57 cases. J. Oral. Pathol. Med. 45, 295–301 (2016).
Choi, Y. S. & Fisher, D. E. UV and melanoma: The TP53 link. Cell Res. 24, 1157–1158 (2014).
Patton, E. E. et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254 (2005).
Akabane, H. & Sullivan, R. J. The future of molecular analysis in melanoma: Diagnostics to direct molecularly targeted therapy. Am. J. Clin. Dermatol. 17, 1–10 (2016).
Pflugfelder, A. et al. Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma”. J. Dtsch. Dermatol. Ges. 11(Suppl 6), 1–116 (2013).
Yamazaki, N. et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: A phase II study. Cancer Sci. 108, 1223–1230 (2017).
Brunssen, A. et al. Long-term relative survival from melanoma in Germany 1997–2013. Melanoma Res. (2018).
Patel, H. et al. Current advances in the treatment of BRAF-mutant melanoma. Cancers (Basel) 12 (2020).
Varada, S. & Mahalingam, M. Mutation stability in primary and metastatic melanoma: What we know and what we don’t. Histol. Histopathol. 30, 763–770 (2015).
Yang, G. et al. Integrated Dataset of mRNA and miRNA Network Associated with the Progression of Metastatic Melanoma by Weighted Gene Co-expression Network Analysis (Scientific Data, 2021).
Nagai, Y. et al. An alternative splicing form of phosphatidylserine-specific phospholipase A1 that exhibits lysophosphatidylserine-specific lysophospholipase activity in humans. J. Biol. Chem. 274, 11053–11059 (1999).
Houben, A. J. & Moolenaar, W. H. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 (2011).
Brindley, D. N., Lin, F. T. & Tigyi, G. J. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta 1831, 74–85 (2013).
Uranbileg, B. et al. Possible involvement of PS-PLA1 and lysophosphatidylserine receptor (LPS1) in hepatocellular carcinoma. Sci. Rep. 10, 2659 (2020).
Emoto, S. et al. Analysis of glycero-lysophospholipids in gastric cancerous ascites. J. Lipid Res. 58, 763–771 (2017).
Iida, Y. et al. Phosphatidylserine-specific phospholipase A1 (PS-PLA1) expression in colorectal cancer correlates with tumor invasion and hematogenous metastasis. Anticancer Res. 35, 1459–1464 (2015).
Kurano, M. et al. Association between serum autotaxin or phosphatidylserine-specific phospholipase A1 levels and melanoma. J. Dermatol. 45, 571–579 (2018).
Aoki, J., Nagai, Y., Hosono, H., Inoue, K. & Arai, H. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim. Biophys. Acta 1582, 26–32 (2002).
Lu, X. et al. Molecular classification and subtype-specific characterization of skin cutaneous melanoma by aggregating multiple genomic platform data. J. Cancer Res. Clin. Oncol. 144, 1635–1647 (2018).
Cancer Genome Atlas, N. Genomic classification of cutaneous melanoma. Cell 161, 1681–1696 (2015).
Imani, S. et al. Novel splicing variant c. 208+2T>C in BBS5 segregates with Bardet–Biedl syndrome in an Iranian family by targeted exome sequencing. Biosci. Rep. 39 (2019).
Wei, C. et al. A novel homozygous variant of GPR98 causes usher syndrome type IIC in a consanguineous Chinese family by next generation sequencing. BMC Med. Genet. 19, 99 (2018).
Cheng, J. et al. Evaluation of PIK3CA mutations as a biomarker in Chinese breast carcinomas from Western China. Cancer Biomark. 19, 85–92 (2017).
Mohapatra, P., Yadav, V., Toftdahl, M. & Andersson, T. WNT5A-induced activation of the protein kinase C substrate MARCKS is required for melanoma cell invasion. Cancers (Basel) 12 (2020).
Kudchadkar, R., Gibney, G. & Sondak, V. K. Integrating molecular biomarkers into current clinical management in melanoma. Methods Mol. Biol. 1102, 27–42 (2014).
Bolander, A. et al. Serological and immunohistochemical analysis of S100 and new derivatives as markers for prognosis in patients with malignant melanoma. Melanoma Res. 18, 412–419 (2008).
Newell, F. et al. Whole-genome sequencing of acral melanoma reveals genomic complexity and diversity. Nat. Commun. 11, 5259 (2020).
Broekaert, S. M. et al. Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res. 23, 763–770 (2010).
Zarei, S. et al. Mutational profile in vulvar, vaginal, and urethral melanomas: Review of 37 cases with focus on primary tumor site. Int. J. Gynecol. Pathol. 39, 587–594 (2020).
Nakawatari, K. et al. Elevated phosphatidylserine-specific phospholipase A1 level in hyperthyroidism. Clin. Chim. Acta 503, 99–106 (2020).
Sawada, T. et al. Serum phosphatidylserine-specific phospholipase A1 as a novel biomarker for monitoring systemic lupus erythematosus disease activity. Int. J. Rheum. Dis. 22, 2059–2066 (2019).
Yang, Q. et al. Phosphatidylserine-specific phospholipase A1 is the critical bridge for hepatitis C virus assembly. Virol. Sin. 34, 521–537 (2019).
Guo, M. et al. Phosphatidylserine-specific phospholipase A1 involved in hepatitis C virus assembly through NS2 complex formation. J. Virol. 89, 2367–2377 (2015).
Yatomi, Y. et al. Lysophospholipids in laboratory medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 94, 373–389 (2018).
Viros, A. et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med. 5, e120 (2008).
Nakamura, K. et al. A novel enzyme immunoassay for the determination of phosphatidylserine-specific phospholipase A(1) in human serum samples. Clin. Chim. Acta 411, 1090–1094 (2010).
King, R. et al. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for MelanomaDiagnosis. Am. J. Pathol. 155, 731–738 (1999).
Weinstein, D., Leininger, J., Hamby, C. & Safai, B. Diagnostic and prognostic biomarkers in melanoma. J. Clin. Aesthet. Dermatol. 7, 13–24 (2014).
Gerami, P. et al. Fluorescence in situ hybridization for distinguishing nevoid melanomas from mitotically active nevi. Am. J. Surg. Pathol. 33, 1783–1788 (2009).
Petersen, E. V. et al. The extracellular matrix-derived biomarkers for diagnosis, prognosis, and personalized therapy of malignant tumors. Front. Oncol. 10, 575569 (2020).
Kim, S. H. et al. Advantages and limitations of current biomarker research: From experimental research to clinical application. Curr. Pharm. Biotechnol. 18, 445–455 (2017).
Grob, J. J. et al. Eighth American Joint Committee on Cancer (AJCC) melanoma classification: Let us reconsider stage III. Eur. J. Cancer 91, 168–170 (2018).
Imani, S. et al. MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget 8, 21362–21379 (2017).
Zou, L. et al. Genomewide copy number analysis of circulating tumor cells in breast cancer patients with liver metastasis. Oncol. Rep. 44, 1075–1093 (2020).
Khan, M. A. et al. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget 6, 19580–19591 (2015).
Livak, K. J. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods 25, 402–408 (2001).
Fan, Y. et al. Epigenetic identification of ADCY4 as a biomarker for breast cancer: An integrated analysis of adenylate cyclases. Epigenomics 11, 1561–1579 (2019).
Wang, M. et al. Bevacizumab combined with apatinib enhances antitumor and anti-angiogenesis effects in a lung cancer model in vitro and in vivo. J. Drug Target 28, 1–9 (2020).
Yang, Q. et al. Anlotinib suppresses colorectal cancer proliferation and angiogenesis via inhibition of AKT/ERK signaling cascade. Cancer Manag. Res. 12, 4937–4948 (2020).
Zamora, J., Abraira, V., Muriel, A., Khan, K. & Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 6, 31 (2006).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Acknowledgements
This work was supported in part by Southwest Medical University Grant and the Research Foundation of the Science and Technology Department of Sichuan Province (No, 18080) and was funded by Saber Imani. The authors would like to express their appreciation to appreciate Department of Oncology and Department of Pathology, Affiliated Hospital of Southwest Medical University, Luzhou, China. We also gratefully acknowledge all authors and co-workers for their contributions.
Author information
Authors and Affiliations
Contributions
Study deign: M.M., M.D.S., and S.I.; investigation and resources: M.D.S.; data collection: G.Y., S.L., M.M., H.H., M.E., and C.Z.; writing-original draft preparation: S.I., S.Z.F., and Q.L.W.; writing-review and editing: Y.D., P.J.K. and S.I.; data interpretation and visualization: P.J.K. and Q.L.W.; supervision: Q.L.W. and S.I.; project administration: G.Y. and S.L.; funding acquisition: S.I.; statistical analysis: M.E. and S.I.. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, G., Liu, S., Maghsoudloo, M. et al. PLA1A expression as a diagnostic marker of BRAF-mutant metastasis in melanoma cancer. Sci Rep 11, 6056 (2021). https://doi.org/10.1038/s41598-021-85595-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85595-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.