Abstract
TP63, a member of the TP53 gene family, is a nuclear marker of myoepithelial cells. Antibody against p63 is frequently used to aid in the diagnosis of prostate carcinoma, as well as in the identification of myoepithelial cells in other tissues including the breast. p63 is also a marker for squamous cell carcinoma. Recently, it was found that all p53 family members are involved in regulating the process of muscle differentiation through the retinoblastoma (RB) protein. Ablation of these p53 family functions blocks the differentiation program and promotes malignant transformation by enabling cooperating oncogenes to transform myoblasts. We therefore studied p63 expression in a number of neoplasms with myogenic differentiation. Immunohistochemical staining for p63 was performed on paraffin sections from 38 rhabdomyosarcomas, five leiomyomas, five leiomyosarcomas, five rhabdomyomas, five rhabdomyomatous Wilms tumors, three normal cardiac muscles, one medullomyoblastoma, one pleuropulmonary blastoma with rhabdomyomatous differentiation, and one teratoma with prominent rhabdomyoblasts. Each case was also stained with desmin. Unlike the nuclear staining scored in myoepithelial cells, only cytoplasmic staining for p63 was considered positive. Of 38 cases of rhabdomyosarcoma, 36 showed cytoplasmic p63 staining; 24 of these showed highlighting of cross-striations superior to that of desmin. In addition, 5/5 rhabdomyomas, 5/5 rhabdomyomatous Wilms tumors, 1/1 pleuropulmonary blastoma with rhabdomyomatous differentiation, 1/1 teratoma with atypical rhabdoblasts, and 1/1 medullomyoblastoma exhibited cytoplasmic p63 staining. Normal cardiac muscle samples (3/3) also demonstrated positive cytoplasmic staining and distinct cross-striations. Smooth muscle tumors exhibited only very focal and faint cytoplasmic staining in 5/5 leiomyomas and 4/5 leiomyosarcomas. Immunoelectron microscopic study of skeletal muscle showed p63 localization to the Z bands of sarcomeres. We conclude that p63 immunostain is a sensitive marker for skeletal muscle differentiation and highlights the cross-striations of strap cells with exceptional definition.
Similar content being viewed by others
Main
The p63 protein, a member of the TP53 gene family, is essential for the development of limbs, craniofacial structures, and several epithelia. p63 is expressed in the basal epithelial cells of different organs, including the breast, skin, uterine cervix, urogenital tract, and prostate. It has also been proposed as a possible marker of stem cells or reserve cells.1 In surgical pathology, antibody to p63, solo or in cocktail combination with other immunohistochemical markers, is frequently used in the diagnosis of prostate carcinoma because normal prostatic glands are lined by p63-positive basal cells, whereas carcinomatous glands lack a basal cell layer.2, 3, 4 p63 immunostaining is also used to support a diagnosis of squamous cell carcinoma in various sites, including the head and neck, lung,5 uterine cervix, and anus,6 as well as metaplastic carcinoma of the breast,7 urothelial carcinoma, sarcomatoid carcinoma,8 and giant cell tumor of bone.9, 10
Recently, it was found that all p53 family members are involved in regulating muscle differentiation through the retinoblastoma (RB) protein. The early stages of muscle differentiation, up to the expression of myogenin, can occur without the p53 family members. However, later steps involving exit from the cell cycle and activation of muscle-specific gene transcription require an active RB protein. The p53 family members function in separate but complementary ways to produce and maintain an active RB protein. p53 is required to induce transcription of the RB gene, whereas p63 and p73 induce the cyclin-dependent kinase inhibitor p57 to maintain RB in an active, hypophosphorylated state. Loss of these p53 family functions by overexpression of DNp73 or mutations in TP53 blocks myogenic differentiation, thereby, allowing cooperating oncogenes to induce neoplastic transformation in myoblasts. Induction of cellular differentiation, therefore, may offer an explanation for the high frequency of TP53 pathway mutations seen in rhabdomyosarcoma patients.11, 12
Rhabdomyosarcoma is the most common soft tissue sarcoma in children.11 Given the important role of p53 family members in the development of this tumor, we hypothesized that the p63 protein might be expressed in these tumors. We further expanded our examination to include a wider variety of tumors with rhabdomyomatous differentiation, including rhabdomyomas and rhabdomyomatous Wilms tumors, as well as smooth muscle tumors and cardiac muscle.
Materials and methods
Immunohistochemistry
A total of 38 rhabdomyosarcomas from 34 patients were selected from the archives of the Department of Pathology at Indiana University School of Medicine. These included 20 embryonal (including seven spindle cell variants), five alveolar, five pleomorphic, four mixed embryonal and alveolar, two with cytodifferentiation, and two unspecified. In addition, five leiomyomas, five leiomyosarcomas, five rhabdomyomas, five rhabdomyomatous Wilms tumors, three cases of normal cardiac muscle, one medullomyoblastoma, one pleuropulmonary blastoma with rhabdomyomatous differentiation, and one teratoma with atypical rhabdoblasts were retrieved. All cases were reviewed, along with appropriate immunostains, and the diagnoses were confirmed. Formalin-fixed paraffin-embedded tissue blocks were cut in 4-μm sections and picked up onto positively charged slides. The sections were then deparaffinized and rehydrated. Antibodies against desmin (Dako, Carpinteria, CA, USA, R606, predilute) and p63 (Dako, M7247, 1:60) were then applied to sections from each case. The p63 antigen was retrieved in 1 mM EDTA buffer, pH8, by heating in a pressure cooker on high for 15 min. The primary antibody was diluted 1:60 and incubated for 30 min with the sections. Detection was accomplished using the LSAB2 method (15 min each, Dako). Desmin antigen was retrieved in Dako's ‘PT Module’ with their high pH-Target Retrieval Solution using Dako's Flex+Mouse avidin–biotin system. Primary antibody and all subsequent incubations were 10 min each. Horseradish peroxidase conjugated to the final reagent was used to develop the brown diaminobenzidine chromagen. The immunostains were then qualitatively reviewed and assessed for the presence or absence of staining. Unlike the nuclear staining scored in myoepithelial cells, only cytoplasmic staining for p63 was considered positive. p63 staining was semiquantitatively scored for intensity on a scale of 0 to 3+, with 0 representing no cytoplasmic staining, 1+ faint cytoplasmic staining, 2+ moderate cytoplasmic staining, and 3+ intense cytoplasmic staining. Percentage of positive cells was not recorded. The presence or absence of visible striations was noted. Desmin was used as a comparison with p63 and was also scored on a scale of 0 to 3+, based on intensity.
Immunoelectron Microscopy
Normal skeletal muscle tissue was fixed with 4% paraformaldehyde in 0.1 M Na cacodylate buffer pH 7.2, dehydrated through a graded series of ethyl alcohols, and embedded in Unicryl (Vector Labs). Thin sections (70–90 nm) were mounted on Formvar/carbon-coated nickel grids. After drying, the grids were floated on drops of 0.05 M glycine for 15 min to quench the aldehydes. After rinsing with 0.1 M phosphate buffer, the grids were placed into the blocking buffer for 30–45 min to block and permeabilize the tissue. The grids were then incubated in p63 antibody (1:5 dilution) at 4°C overnight, rinsed with the incubation buffer, and floated on drops of the secondary antibody with attached 10 nm gold particles (Aurion, Electron Microscopy Sciences, Hatfield, PA, USA) for 2 h at room temperature. The grids were rinsed again in buffer and placed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 5 min. The grids were finally rinsed in distilled water, allowed to dry, and stained for contrast with uranyl acetate. The skeletal muscle samples were then viewed with a Tecnai Bio (Tecnai G2 Spirit Bio (Twin)) transmission electron microscope (FEI, Hillsboro, OR, USA) and photographed (AMT XR60).
Results
Immunohistochemistry
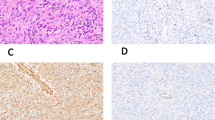
Of 38 rhabdomyosarcomas, 36 (95%) showed cytoplasmic p63 staining; 24 had intense staining (3+); nine moderate (2+); and three faint (1+) (Table 1). p63 clearly highlighted the cross-striations characteristic of skeletal muscle differentiation in 24 (63%) of the 38 cases (Figures 1a and b). In comparison, cytoplasmic desmin staining was seen in 35 of 38 rhabdomyosarcomas, but only two displayed somewhat distinct cross-striations. In addition, 5/5 rhabdomyomas (three 3+, one 2+, one 1+), 5/5 rhabdomyomatous Wilms tumors (four 3+, one 1+), 1/1 pleuropulmonary blastoma with rhabdomyomatous differentiation (3+), 1/1 teratoma with atypical rhabdoblasts (2+), and 1/1 medullomyoblastoma (3+) exhibited cytoplasmic p63 staining. Several of the tumor samples included adjacent normal skeletal muscle tissues that showed intense cytoplasmic immunoreactivity with prominence of the cross-striations. Normal cardiac muscle samples (3/3, two 3+, one 2+) also demonstrated positive cytoplasmic staining and distinct cross-striations (Figures 1c–f). Smooth muscle tumors exhibited only very focal and faint cytoplasmic staining in 5/5 leiomyomas (all 1+) and 4/5 leiomyosarcomas (one 3+, three 2+, one 0).
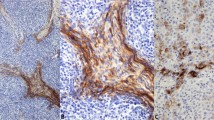
Sections where the strap cells could be identified on H&E preparation, demonstrated parallel desmin and p63 immunoreactivity; however, p63 highlighted the cross-striations of the strap cells, with a degree of definition not seen on the desmin immunostains (Figure 2). Despite the fact that desmin was a poor marker for cross-striations, it showed diffuse and usually intense immunoreactivity in most cases (Table 1).
Immunoelectron Microscopy
Skeletal muscle sections displayed p63 localization primarily to the Z lines of the sarcomeres (Figure 3).
Discussion
p63 immunostain is a sensitive marker for skeletal and cardiac muscle differentiation, and highlights the cross-striations of strap cells with exceptional definition, much superior to desmin. The high frequency of distinct cross-striations on immunohistochemistry, in conjunction with our immunoelectron microscopy study supports that p63 localizes to the Z lines of skeletal and cardiac muscle. This explains why smooth muscle, which lacks cross-striations and Z lines, does not stain as intensely with p63. Desmin has been reported to be concentrated in the bundles of intermediate filaments connecting Z lines to adjacent myofibrils. In addition to being present between myofibrils, desmin filaments are also found within myofibrils, which can explain its more diffuse staining. Smooth muscle cells also contain desmin intermediate filaments and stain with desmin immunohistochemistry.13
It should be noted that rhabdomyosarcomas composed of more primitive small cells and fewer strap cells, did not stain as intensely with p63 as those with more abundant and prominent strap cells. Although many of the cases demonstrated remarkable definition and frank beauty, there were also those cases that displayed only faint staining, which was sometimes difficult to differentiate from insignificant background staining.
While, in this study, only cytoplasmic staining was the subject of our analysis, we did observe nuclear staining in a small proportion of tumors. The significance of this nuclear positivity is unclear and will require further studies to elucidate.
p63 is a widely available and frequently used antibody, with many applications in general surgical pathology laboratories. We propose an additional application for p63 as a potential marker for striated muscle differentiation. It may be used in conjunction with the current skeletal muscle markers, desmin, myogenin, and myf-1. p63 may also be, especially useful in distinguishing skeletal muscle tumors from smooth muscle tumors. For example, many paratesticular rhabdomyosarcomas display spindle cell morphology, and leiomyosarcoma may enter into the differential diagnosis in this situation. Desmin stains both skeletal and smooth muscle, and therefore cannot be used to distinguish the two. However, p63 could potentially be used to aid in this differential diagnosis, as it may highlight cross-striations not seen with desmin immunohistochemistry (Figure 4).
Comparison of spindle cell rhabdomyosarcoma (a–c, H&E, 800 × ) and leiomyosarcoma (d–f, H&E, 800 × ). Both entities show immunoreactivity for desmin (b and e, 800 × ), but p63-stained cross-striations are visible only in the spindle cell rhabdomyosarcoma (c, 800 × ). Cross-striations are not seen in the leiomyosarcoma stained with p63 (f, 800 × ).
Our findings of p63 expression in skeletal muscle and tumors with skeletal muscle differentiation lend support to recent reports linking the p53 family of proteins to myogenic differentiation and rhabdomyosarcoma development.11 Whether or not the p63 immunostain becomes a highly used marker for skeletal muscle differentiation remains to be seen.
References
Barbareschi M, Pecciarini L, Cangi MG, et al p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol 2001;25:1054–1060.
Shah RB, Zhou M, LeBlanc M, et al Comparison of the basal cell-specific markers, 34betaE12 and p63, in the diagnosis of prostate cancer. Am J Surg Pathol 2002;26:1161–1168.
Weinstein MH, Signoretti S, Loda M . Diagnostic utility of immunohistochemical staining for p63, a sensitive marker of prostatic basal cells. Mod Pathol 2002;15:1302–1308.
Tacha DE, Miller RT . Use of p63/P504S monoclonal antibody cocktail in immunohistochemical staining of prostate tissue. Appl Immunohistochem Molecul Morphol 2004;12:75–78.
Reis-Filho JS, Simpson PT, Martins A, et al Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch 2003;443:122–132.
Owens SR, Greenson JK . Immunohistochemical staining for p63 is useful in the diagnosis of anal squamous cell carcinomas. Am J Surg Pathol 2007;31:285–290.
Koker MM, Kleer CG . p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol 2004;28:1506–1512.
Lewis JS, Ritter JH, El-Mofty S . Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Mod Pathol 2005;18:1471–1481.
Dickson BC, Li S-Q, Wunder JS, et al Giant cell tumor of bone express p63. Mod Pathol 2008;21:369–375.
Lee C-H, Espinosa I, Jensen KC, et al Gene expression profiling identifies p63 as a diagnostic marker for giant cell tumor of the bone. Mod Pathol 2008;21:531–539.
Cam H, Griesmann H, Beitzinger M, et al p53 family members in myogenic differentiation and rhabdomyosarcoma development. Cancer Cell 2006;10:281–293.
Ikawa S, Nakagawara A, Ikawa Y . p53 family genes: structural comparison, expression and mutation. [see comment]. Cell Death Differ 1999;6:1154–1161.
Yang YG, Makita T . Immunocytochemical localization of desmin in skeletal muscle of swine. J Vet Med Sci 1995;57:475–479.
Acknowledgements
The authors would like to thank Tracey Bender for her assistance with editing and formatting this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented, in part, at the 85th annual meeting of the American Association of Neuropathologists, San Antonio, TX, 11–14 June 2009 and at the 99th annual meeting of the United States and Canadian Academy of Pathology, Washington, DC, 20–26 March 2010.
Rights and permissions
About this article
Cite this article
Martin, S., Temm, C., Goheen, M. et al. Cytoplasmic p63 immunohistochemistry is a useful marker for muscle differentiation: an immunohistochemical and immunoelectron microscopic study. Mod Pathol 24, 1320–1326 (2011). https://doi.org/10.1038/modpathol.2011.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.89
Keywords
This article is cited by
-
Tissue-specific expression of p73 and p63 isoforms in human tissues
Cell Death & Disease (2021)
-
Canalicular Adenoma: A Clinicopathologic and Immunohistochemical Analysis of 67 Cases with a Review of the Literature
Head and Neck Pathology (2015)