Abstract
Epithelial–mesenchymal transition is characterized by a loss of cell adhesion and increased cell mobility due to cells gaining a mesenchymal phenotype. During the epithelial–mesenchymal transition process, tumor cells are expected to lose their epithelial phenotype and gradually and sequentially acquire a mesenchymal phenotype. Epithelial–mesenchymal transition is a dynamic and reversible process, which has been observed in patient tissues to display a wide spectrum of phenotypes. However, very little is known about the clinical significance of the different phenotypes of the epithelial–mesenchymal transition. Based on the expression pattern of various epithelial–mesenchymal transition-related proteins, we divided 168 esophageal squamous cell carcinomas into different phenotypes, including complete type; incomplete type, including hybrid type and null type; and a wild type. The clinical significance of each phenotype was investigated. Of the 168 cases, 31 were categorized as complete type, 53 as incomplete type (hybrid type, 26 cases; null type, 27 cases), and 84 as wild type. Epithelial–mesenchymal transition phenotype was significantly associated with tumor size (P=0.021), differentiation (P=0.001), and invasion depth (P<0.001). Overall survival and disease-free survival rates were significantly worse in the complete type, better in the incomplete type, and best in the wild type. Within the incomplete type group, the hybrid type survival curve was similar to that of the complete type, whereas the overall survival of the null type was similar to the wild type. Complete type had a noticeable poorer prognostic effect on survival in patients with early invasion (pT≤2) than it had on survival among patients with advanced invasion (pT≥3). The complete phenotype was an independent prognostic factor for both overall (P=0.009) and disease-free survival (P<0.001). In conclusion, classification of epithelial–mesenchymal transition phenotypes has novel clinical implications, and identification of a specific phenotype might provide a tool to better stratify and predict patient outcomes.
Similar content being viewed by others
Main
The process of epithelial cells changing to mesenchymal cells, referred to as the epithelial–mesenchymal transition, is characterized by a loss of cell adhesion and increased cell mobility due to cells gaining a mesenchymal phenotype. Epithelial–mesenchymal transition is a normal developmental process for mesoderm and neural tube formation.1 Although the concept of epithelial–mesenchymal transition in cancer is still controversial, epithelial–mesenchymal transition has been implicated in a number of epithelial cancers and has been shown to correlate with the metastatic potential of cancers.1, 2 Although epithelial–mesenchymal transition has been documented in a large number of cancers, most studies have used in vitro systems that employ cell lines and focused on the detailed mechanism of epithelial–mesenchymal transition, identifying a number of transcription factors and signaling pathways involved. Therefore, little is known about the exact and detailed pattern of epithelial–mesenchymal transition in patient cancer tissues or the clinical importance of epithelial–mesenchymal transition itself in each type of cancer.2
During the epithelial–mesenchymal transition process, tumor cells are expected to lose their epithelial phenotype and gradually and sequentially acquire a mesenchymal phenotype. However, due to the complexity of epithelial–mesenchymal transition and the diversity of cancer cells and their surrounding microenvironment, epithelial–mesenchymal transition actually observed in patient tissues has shown a wide spectrum of phenotypes. In addition, because of the dynamic and reversible nature of epithelial–mesenchymal transition, numerous advanced carcinomas that adopt some mesenchymal features retain the characteristics of well-differentiated epithelial cells.3, 4 In vivo, epithelial–mesenchymal transition phenotypes include the extremes, ranging from an epithelial phenotype to a complete mesenchymal phenotype with a variety of intermediate sub-phenotype combinations between these extremes. Examples of intermediate sub-phenotypes include the ‘hybrid type’ that is a mixed epithelial and mesenchymal phenotype and the ‘null type’ that can be defined as the loss of the epithelial phenotype without acquisition of mesenchymal phenotype. Thus, we propose that carcinomas can be classified into the following four specific types of epithelial–mesenchymal transition: (1) complete type characterized by loss of the epithelial phenotype with acquisition of the mesenchymal phenotype, (2) incomplete type 1 (hybrid type) characterized by a tumor showing both mesenchymal and epithelial phenotypes, (3) incomplete type 2 (null type) defined by loss of the epithelial phenotype without acquisition of a mesenchymal phenotype, and (4) wild type characterized by a tumor with no evidence of epithelial–mesenchymal transition.
Esophageal squamous cell carcinoma is one of the most aggressive cancers, and epithelial–mesenchymal transition has been reported to be associated with more aggressive tumor behavior in esophageal squamous cell carcinoma.5, 6, 7, 8 Generally, the loss of E-cadherin is considered a common indicator for the onset of epithelial–mesenchymal transition, and it has been shown that Snail-dependent E-cadherin repression is one of the most important mechanisms for E-cadherin loss in esophageal squamous cell carcinoma.5, 8 However, there are some limitations in these studies due to the small number of cases investigated, and very little is known about the clinical significance of detailed epithelial–mesenchymal transition sub-phenotypes in esophageal squamous cell carcinoma. Therefore, in this study, we divided epithelial–mesenchymal transition phenotype into sub-phenotypes based specifically on the expression pattern of various epithelial–mesenchymal transition-related proteins and investigated the clinical significance of each sub-phenotype in a large series of resected esophageal squamous cell carcinoma samples.
Materials and methods
Tissue Samples
We included a total of 168 formalin-fixed and paraffin-embedded tumor samples from patients who underwent curative surgical resection for primary esophageal squamous cell carcinoma at the Samsung Medical Center between 1995 and 2008. All patients provided written informed consent according to institutional guidelines. No patients received preoperative chemotherapy or radiotherapy. Clinical and pathologic reports were reviewed for age, sex, tumor size, histological grade, invasion depth (pT), nodal status (pN), and distant metastasis (pM). The histological grade was based on the WHO classification. The pTNM classification was applied according to guidelines from the 2010 American Joint Committee on Cancer staging manual.9 Of the 168 tumors, 34 cases were in T1, 29 were in T2, 95 were in T3, and 10 were in T4. For N classification, 57 cases were in N0, 41 were in N1, 31 were in N2, and 27 in N3. The average number of reviewed lymph nodes was 43.6 per case and ranged from 4 to 134. There were 12 cases with <20 examined regional nodes that were likely not adequately evaluated for lymph node status. Therefore, these cases were excluded from the N classification. There were 21 patients with distant metastasis (M1).
Tissue Microarray and Immunohistochemistry
Hematoxylin and eosin (HE)-stained tissues were reviewed to confirm the histological diagnosis and to select representative areas for immunostaining. A cylindrical core (3 mm in diameter) was removed from formalin-fixed and paraffin-embedded tissue blocks corresponding to the HE slides to construct the tissue microarray. Sectioning of microarray blocks produced 4 μm thick sections after completion of the tissue array.
Microslide tissue sections were deparaffinized with xylene, hydrated using a diluted alcohol series, and immersed in 0.3% H2O2 in methanol to quench endogenous peroxidase activity. Sections were then microwaved for 15 min in 10 mM citrate buffer (pH 6.0) for antigen retrieval. To reduce non-specific staining, each section was blocked with 4% bovine serum albumin in PBS with 0.1% Tween 20 (PBST) for 30 min. Sections were then incubated with anti-fibronectin (dilution: 1:100, BioGenex, San Ramon, CA, USA), anti-smooth muscle actin (dilution: 1:100, DAKO, Glostrup, Denmark), anti-vimentin (dilution: 1:200, DAKO), anti-E-cadherin (dilution: 1:50, Invitrogen, Carlsbad, CA, USA), and anti-Snail antibody (dilution: 1:50, Abcam, Cambridge, UK) in PBST containing 3 mg/ml goat globulin (Sigma) for 60 min at room temperature, followed by three successive washes with buffer. Sections were then incubated with an anti-mouse/rabbit antibody (Envision plus, Dako, Carpinteria, CA, USA) for 30 min at room temperature. The chromogen used was 3,3′-diaminobenzidine (Dako). Sections were counterstained with Meyer's hematoxylin. Omitting the primary antibody provided negative controls for immunostaining using normal mouse and rabbit serum.
Evaluation of Immunohistochemical Staining Results
In this study, we used the scoring method of Sinicrope et al,10 which was applied for the evaluation of both immunohistochemical staining intensity and the proportion of stained epithelial cells. Staining intensity was further classified as follows: (1) weak, (2) moderate, or (3) strong. Positive cells were quantified as a percentage of the total number of epithelial cells and assigned to one of the following five categories: 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; or 4, >75%. The percentage of epithelial cell positivity and staining intensity were multiplied to generate an immunoreactivity score for each case. For example, if the staining intensity was strong (3 points) and the percentage of positive cells was >75% (4 points), then the immunoreactivity score was calculated as 3 × 4=12. On the other hand, if the staining intensity was weak (1 point) and the percentage of positive cells was between 5 and 25% (1 point), then the immunoreactivity score was calculated as 1 × 1=1. As a result, immunoreactivity score values ranged from 0 to 12. Two pathologists (COS and SHK), who were blinded to patient outcomes, independently examined and scored each lesion. Differences in interpretation were resolved by consensual agreement.
As there are no absolute criteria for the immunopositivity of each antibody used in this study, we selected a cutoff point to designate immunopositivity that was most meaningful with respect to prognosis using the Kaplan–Meier method with long-rank test. This method considered the following values as positive: fibronectin (immunoreactivity score ≥3), smooth muscle actin (immunoreactivity score ≥2), vimentin (immunoreactivity score ≥5), E-cadherin, and Snail (immunoreactivity score ≥4).
Statistical Analysis
Correlations were examined using Pearson's χ2 or Fisher's exact test, as appropriate. Disease-free survival and overall survival were determined using the Kaplan–Meier method, and survival curves were compared using the log-rank test. Survival was measured from the date of surgery to May 2009. Postoperative follow-up data were obtained from all patients, with a median follow-up period of 33 months (range, 1–155 months). The Cox Proportional Hazard Model was used to evaluate the association between clinicopathologic factors and survival. The hazard ratio and its 95% confidence interval were assessed for each factor. All tests were two-sided, and P-values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 18 (SPSS, Chicago, IL, USA).
Results
Expression of Epithelial–Mesenchymal Transition-Related Proteins in Esophageal Squamous Cell Carcinoma
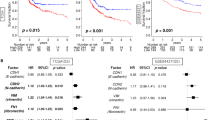
Reduced expression of E-cadherin was observed in 58/168 (35%) samples. Overall, 78 (46%) cases showed Snail expression in the tumor cell nuclei. Cytoplasmic expression of fibronectin, smooth muscle actin, and vimentin was observed in 31 (18%), 28 (17%), and 29 (17%) cases, respectively. Snail expression was significantly correlated with reduced expression of E-cadherin (P=0.003) and overexpression of smooth muscle actin (P=0.004) and vimentin (P=0.007) (Table 1). However, Snail expression was not associated with fibronectin expression (P=0.150). In the univariate survival analysis, all epithelial–mesenchymal transition-related proteins except for Snail were significantly correlated with poor overall survival (Figure 1). Although cancers with Snail expression tended to have a poor prognosis, the relationship was not statistically significant (P=0.098).
Classification of Epithelial–Mesenchymal Transition Phenotype and Clinicopathological Features
Based on the combined results from our analysis of expression of epithelial (E-cadherin) and mesenchymal (fibronectin, smooth muscle actin, and vimentin) markers, we divided the 168 cases of esophageal squamous cell carcinoma into the following three groups (Figure 2a): complete phenotype (positive for any mesenchymal marker and a loss of E-cadherin), wild type (preserved E-cadherin expression and negative for mesenchymal markers), and incomplete phenotype (neither complete nor wild type). The incomplete type was again subdivided into the following two groups based on phenotype: the hybrid type (positive for both E-cadherin and any mesenchymal marker expression) and the null type (negative for both E-cadherin and mesenchymal markers). Representative cases for these detailed sub-phenotypes are shown in Figure 2b. Of the 168 cases, we identified 31 (18%) cases of complete type, 53 (32%) of incomplete type (hybrid, 26 cases; null type, 27 cases), and 84 (50%) cases of wild type. There was a significant correlation between epithelial–mesenchymal transition phenotype and Snail expression (P<0.001; Figure 2c). In addition, epithelial–mesenchymal transition phenotype was significantly associated with major clinicopathological parameters, including tumor size (P=0.021), histological differentiation (P=0.001), and invasion depth (P<0.001) (Table 2). Of the 168 cases, 84% of the 31 complete type and 72% of the 53 incomplete type cases showed advanced invasion (≥pT3), whereas 49% of the 84 wild-type cases showed advanced invasion.
(a) Classification of epithelial–mesenchymal transition (EMT) phenotype for 168 esophageal squamous cell carcinomas according to their E-cadherin, fibronectin, smooth muscle actin (SMA), and vimentin expression patterns. (b) Representative cases of each EMT phenotype with their corresponding immunostaining. (b, inset) Nuclear expression of Snail. (c) Correlation between EMT phenotype and Snail expression. Note that Snail expression increased according to EMT phenotype (P<0.001 for trend).
Impact of Epithelial–Mesenchymal Transition Phenotype on Patient Survival
Epithelial–mesenchymal transition phenotype survival rates were compared against each other. There was a significant difference in survival rates between each group in terms of both overall survival (P<0.05) (Figure 3a) and disease-free survival (P<0.05) (Figure 3b). Overall survival and disease-free survival rates were worst in the complete type, better in the incomplete type, and best in the wild-type group. The 5-year overall survival rates of the complete, incomplete, and wild-type groups were 26, 43, and 61%, respectively. Between the incomplete type subgroups, the hybrid type showed a poorer prognosis than the null type, but the difference was not statistically significant for either overall survival (P=0.052; Figure 3c) or disease-free survival (P=0.166; Figure 3d). However, the survival curve for the hybrid type was similar to that of complete type, whereas overall survival for the null type was similar to the wild type. When patients were stratified by invasion depth, complete type had a noticeably greater effect on survival in patients with early invasion (pT≤2) than in patients with advanced invasion (pT≥3) (Figure 4). To confirm the epithelial–mesenchymal transition phenotype as an independent prognostic factor, we performed a multivariate Cox Proportional Hazards Model analysis, including age, gender, tumor size, differentiation, pT classification, pN classification, and distant metastasis as confounding factors. In this multivariate analysis, the complete phenotype was an independent prognostic factor for both overall (P=0.009) and disease-free survival (P<0.001) (Table 3).
Discussion
In this study, we classified epithelial–mesenchymal transition phenotype based on epithelial–mesenchymal transition-related protein expression patterns, and found that the phenotype had significant prognostic value. In addition, expression of mesenchymal markers was more important for outcome prediction than repression of epithelial markers, which seems reasonable because the acquisition of mesenchymal phenotype enhances motility and invasion of cancer cells into the surrounding tissue. This may explain why complete type was significantly associated with increased tumor invasion (high pT classification) in this study. Furthermore, we demonstrated that complete type had a noticeably greater effect on survival in early esophageal squamous cell carcinoma than in advanced carcinoma. However, in this study epithelial–mesenchymal transition phenotype did not correlate with lymph node metastasis or distant metastasis.
Snail is a known direct repressor of E-cadherin, promoting epithelial–mesenchymal transition.11 This study demonstrated that Snail overexpression significantly correlated with reduced expression of E-cadherin. In addition, we showed that Snail expression was significantly associated with mesenchymal markers, such as vimentin and smooth muscle actin. Usami et al5 reported similar results; their data showed that nuclear Snail expression correlated with reduced expression of E-cadherin and vimentin overexpression in esophageal squamous cell carcinoma. Using double immunofluorescence labeling in oral squamous cell carcinomas, Franz et al12 reported that most Snail-positive cells also expressed smooth muscle actin. In this study, Snail expression did not correlate with fibronectin expression. However, reduced expression of E-cadherin was significantly correlated with overexpression of fibronectin (data not shown). Recently, Jethwa et al13 reported that overexpression of Slug induced fibronectin in an esophageal adenocarcinoma cell line. These findings suggest that molecules other than Snail regulate fibronectin expression.
One important issue of epithelial–mesenchymal transition in solid cancers is the presence of the incomplete phenotype. In this study, we identified a significant number of incomplete phenotype cases that showed intermediate prognosis between the complete type and wild-type groups. Recently, Usami et al5 categorized 72 esophageal cancers as either a complete phenotype, including tumors positive for vimentin immunoreactivity independent of epithelial marker expression (including E-cadherin and claudins); an incomplete phenotype, including tumors negative for both vimentin and E-cadherin, claudin-1 and claudin-7; or wild type, including tumors negative for vimentin expression but preserved expression of E-cadherin, claudin expression, or both. With these classifications, they briefly reported that the complete phenotype was significantly associated with lymph node metastasis and tumor stage in 72 esophageal squamous cell carcinoma cases. They also identified a small portion of cancers that had both epithelial features and mesenchymal characteristics with vimentin expression, which corresponds to the hybrid type incomplete epithelial–mesenchymal transition in our study. Usami et al classified this hybrid type into their complete epithelial–mesenchymal transition category, whereas we classified the hybrid type into the incomplete epithelial–mesenchymal transition category along with the null type. Our approach was similar to the work of Hugo et al2 that indicated that the hybrid state was a result of incomplete epithelial–mesenchymal transition. For our classification, we found that the hybrid type behavior was closer to that of the complete type, whereas behavior of the null type was more similar to the wild type.
Loss of E-cadherin expression is a well-documented condition for invasiveness;14, 15, 16, 17, 18, 19, 20 however, this result is debatable.3, 4 Our work demonstrated that the loss of E-cadherin itself, without a mesenchymal phenotype, may not be associated with invasive behavior, whereas tumors with a mesenchymal phenotype, regardless of E-cadherin expression, showed aggressive behavior in esophageal squamous cell carcinoma. Recently, Uchikado et al21 reported that the patients with esophageal squamous cell carcinoma who were positive for Slug expression had deeper tumor invasion and worse prognosis in the E-cadherin preserved group. These E-cadherin (+) and Slug (+) cases may be related to the hybrid type of epithelial–mesenchymal transition because Slug is also an important transcription factor involved in epithelial–mesenchymal transition.22 Furthermore, Natsugoe et al23 also described that some epithelial–mesenchymal transition patients with preserved E-cadherin expression had a poor prognosis, and the overall survival rate was better in patients with negative Snail expression than in those with positive Snail expression in the preserved E-cadherin group. These results could be explained if the ones with poor prognosis in the group with preserved E-cadherin are associated with the cases that were classified as the hybrid type of epithelial–mesenchymal transition in our study.
A limitation of this study is that we did not evaluate all mesenchymal and epithelial markers for classification of epithelial–mesenchymal transition phenotype. There are other known mesenchymal and epithelial markers related to epithelial–mesenchymal transition, such as the mesenchymal markers thrombospondin, N-cadherin, Tenascin C, and MMP3 and the epithelial markers cytokeratin, occludin, and claudin.24 However, the proteins selected in this study were the most important and representative proteins for epithelial–mesenchymal transition-related phenotypes.
In conclusion, classification of epithelial–mesenchymal transition phenotype has novel implications for epithelial–mesenchymal transition markers in esophageal squamous cell carcinoma, and identification of specific phenotypes in tumor samples might provide a tool to better stratify and predict patient outcomes.
References
Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871–890.
Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol 2007;213:374–383.
Christiansen JJ, Rajasekaran AK . Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006;66:8319–8326.
Lundgren K, Nordenskjold B, Landberg G . Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer 2009;101:1769–1781.
Usami Y, Satake S, Nakayama F, et al. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol 2008;215:330–339.
Cai Z, Wang Q, Zhou Y, et al. Epidermal growth factor-induced epithelial-mesenchymal transition in human esophageal carcinoma cells--a model for the study of metastasis. Cancer Lett 2010;296:88–95.
Isohata N, Aoyagi K, Mabuchi T, et al. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. Int J Cancer 2009;125:1212–1221.
Natsugoe S, Uchikado Y, Okumura H, et al. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma. Oncol Rep 2007;17:517–523.
Edge SB, Byrd DR, Compton CC, et al. American Joint Commission on Cancer Staging Manual. 7th edn. Springer: New York, 2010, pp 103–111.
Sinicrope FA, Ruan SB, Cleary KR, et al. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res 1995;55:237–241.
Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000;2:84–89.
Franz M, Spiegel K, Umbreit C, et al. Expression of Snail is associated with myofibroblast phenotype development in oral squamous cell carcinoma. Histochem Cell Biol 2009;131:651–660.
Jethwa P, Naqvi M, Hardy RG, et al. Overexpression of Slug is associated with malignant progression of esophageal adenocarcinoma. World J Gastroenterol 2008;14:1044–1052.
Nakanishi Y, Ochiai A, Akimoto S, et al. Oncology 1997;54:158–165.
Tamura S, Shiozaki H, Miyata M, et al. Decreased E-cadherin expression is associated with haematogenous recurrence and poor prognosis in patients with squamous cell carcinoma of the oesophagus. Br J Surg 1996;83:1608–1614.
Inada S, Koto T, Futami K, et al. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor). Surg Today 1999;29:493–503.
Jian WG, Darnton SJ, Jenner K, et al. Expression of E-cadherin in oesophageal carcinomas from the UK and China: disparities in prognostic significance. J Clin Pathol 1997;50:640–644.
Pomp J, Blom J, van Krimpen C, et al. E-cadherin expression in oesophageal carcinoma treated with high-dose radiotherapy; correlation with pretreatment parameters and treatment outcome. J Cancer Res Clin Oncol 1999;125:641–645.
Sasaki K, Natsugoe S, Ishigami S, et al. Significance of twist expression and its association with E-cadherin in esophageal squamous cell carcinoma. J Exp Clin Cancer Res 2009;28:158.
Nair KS, Naidoo R, Chetty R . Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol 2005;58:343–351.
Uchikado Y, Natsugoe S, Okumura H, et al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res 2005;11:1174–1180.
Alves CC, Carneiro F, Hoefler H, et al. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci 2009;14:3035–3050.
Natsugoe S, Uchikado Y, Okumura H, et al. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma. Oncol Rep 2007;17:517–523.
Turley EA, Veiseh M, Radisky DC, et al. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol 2008;5:280–290.
Acknowledgements
This study was supported by grants from the Korea Healthcare Technology R&D project of the Ministry of Health, Welfare & Family Affairs (A084366) and (A092255) Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sung, C., Park, CK. & Kim, SH. Classification of epithelial–mesenchymal transition phenotypes in esophageal squamous cell carcinoma is strongly associated with patient prognosis. Mod Pathol 24, 1060–1068 (2011). https://doi.org/10.1038/modpathol.2011.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.59
Keywords
This article is cited by
-
Expression of fibronectin in esophageal squamous cell carcinoma and its role in migration
BMC Cancer (2018)
-
Epithelial Mesenchymal Transition (EMT) in Metastatic Breast Cancer in Omani Women
Cancer Microenvironment (2017)
-
Prognostic significance of epithelial–mesenchymal transition-related markers in extrahepatic cholangiocarcinoma: comprehensive immunohistochemical study using a tissue microarray
British Journal of Cancer (2014)
-
Snail1 correlates with patient outcomes in E-cadherin-preserved gastroesophageal junction adenocarcinoma
Clinical and Translational Oncology (2014)