Abstract
Immunoglobulin A (IgA) is commonly recognized as the most prevalent antibody (Ab) at mucosal sites with an important role in defense by shielding mucosal surfaces from invasion by pathogens. However, its potential to both actively dampen excessive immune responses or to initiate potent proinflammatory cellular processes is less well known. Interestingly, either functional outcome is mediated through interaction with the myeloid IgA Fc receptor FcαRI (CD89). Monomeric interaction of IgA with FcαRI triggers inhibitory signals that block activation via other receptors, whereas multimeric FcαRI crosslinking induces phagocytosis, reactive oxygen species production, antigen presentation, Ab-dependent cellular cytotoxicity, and cytokine release. Thus, FcαRI acts as a regulator between anti- and proinflammatory responses of IgA. As such, the biology of FcαRI, and its multifaceted role in immunity will be the focus of this review.
Similar content being viewed by others
Introduction
Immunoglobulin A (IgA) is the predominant antibody (Ab) class present in mucosal areas, where it has a key role in mucosal defense.1 Mucosal surfaces represent a vast interface of over 400 m2 that protects internal tissues from external influences. They are continuously exposed to inhaled or ingested antigens and pathogens, and are colonized by a commensal microbiota, which must be prevented from penetrating the underlying tissues. As such, mucosal homeostasis requires a delicate balance between avoiding disproportionate responses against innocuous antigens, whereas at the same time effective immunological responses against pathogenic microorganisms must be maintained.2 Interestingly, depending on the circumstances, IgA is involved in anti- as well as proinflammatory responses, although the view of IgA as an anti- or non-inflammatory Ab commonly prevails.3, 4
At mucosal sides, IgA is produced in the LP by local plasma cells as dimeric molecules (dimeric IgA; dIgA), containing a joining J chain. It serves as an intermediary, and can bind to the pIgR that is expressed on the basolateral membrane of epithelial cells. Subsequently, it is transported through the epithelial cells and released into the lumen as SIgA.5, 6, 7 Apical cleavage of pIgR ensures continual attachment of a part of this receptor—referred to as SC—which renders increased stability and prevents rapid breakdown of SIgA in the hostile gut lumen milieu. In addition, 1–3 mg ml−1 IgA is present in the circulation as a monomer, and as such it is the second prevalent Ab in serum.
Multiple types of cellular IgA receptors have currently been characterized. In addition to pIgR,8 Fcα/μ receptors,9 asialoglycoprotein receptors,10 transferrin receptors (CD71),11 SC receptors,12 and M-cell receptors13 have been described that can bind the IgA Fc tail, carbohydrate side chains or accessory molecules such as the J chain and SC. The functions of a number of these receptors have not yet been completely elucidated.
In addition, in humans, a myeloid Fc receptor for IgA (FcαRI; CD89) has been described. Thus far, FcαRI gene homologs have been identified in primates, horses, cattle, hamsters, gerbils, and rats, but not in mice, which is attributable to a gene translocation.14, 15, 16, 17, 18, 19 The absence of FcαRI in mice, which are frequently used for experimental work, has likely influenced the earlier dogma of IgA as an anti-inflammatory Ab class. However, a main role for FcαRI in immune defense is supported, as bacterial evolution has led to the development of proteins that interfere with IgA binding to FcαRI (e.g., IgA-binding M-like proteins Arp4, Sir22, B-antigen, and members of the staphylococcal superantigen-like proteins family),20 resulting in an important evasion strategy for pathogens to escape IgA-mediated phagocytosis. In this review we will therefore address the biology, function and therapeutic potential of FcαRI.
FcαRI: Genetics, Transcripts, and Protein Structure
FcαRI is a member of the Fc receptor Ig superfamily, although distinct differences can be observed compared with other Fc receptors. For instance, the FcαRI gene is located on chromosome 19 (at 19q13.4) and lies within the so-called leukocyte receptor cluster (LRC),21, 22 whereas other FcR genes, like FcγRs and FcɛRI, map on chromosome 1.23, 24 The human LRC includes no other Fc receptor genes, but instead it encodes killer cell immunoglobulin-like receptors and leukocyte Ig-like receptors. FcαRI shows more amino acid (aa) sequence similarities with these receptors than with other human Fc receptors.25 In addition, the murine paired Ig-like receptor-A that was identified in mice on the basis of the homology with FcαRI, shares sequence similarity with both human FcαRI and killer cell immunoglobulin-like receptors.26
FcαRI consists of two extracellular (EC) Ig-like domains of each 206 aa, a 19-aa transmembrane region (TM), which is crucial for association to the signaling FcR γ-chain, and a short (41 aa) cytoplasmic tail. The two EC domains are folded with an angle of approximately 90° to each other. The FcαRI gene (FCAR) coding for this protein consists of five exons. The first two exons encode the leader peptide (S1, 34 bp, and S2, 36 bp), whereas exons 3 (291 bp) and 4 (288 bp) code for EC1 and EC2, respectively. The transmembrane and intracellular domains are both encoded by exon 5 (215 bp).27, 28, 29 Furthermore, a 78-bp insertion sequence (S3) between S2 and EC1 has been reported.30 The FcαRI promoter was identified in a 929-bp fragment of FcαRI 5′-flanking sequence, in which tissue-specific gene expression is regulated by 259 bp, proximal to the translation initiation site.31 The sequence between 59 and 197 bp, downstream of the major transcription start site, is essential for promoter activity and contains multiple potential binding sites for transcription factors ((C/EBP) binding sites, nuclear factor-κB binding site, Spl site, Ets family protein consensus binding site, and a Myb binding site), which have been reported to function in myeloid-specific gene expression.32, 33 The core protein has a predicted molecular weight of 30 kDa. However, FcαRI that is expressed on the cell surface has an apparent molecular weight between 50 and 75 kDa, with the exception of eosinophil FcαRI, which runs between 70 and 100 kDa. This heterogeneous pattern of surface-expressed FcαRI is a consequence of both N- and O-glycosylation.34, 35, 36
Several isoforms have been described in primary cells in addition to the full-length FcαRI protein. FcαRIa.2 bears deletion of 66 aa in EC2, and it is the only isoform expressed on alveolar macrophages, which might have a physiological relevance in IgA-mediated host defense in the lung.37 In addition, the isoform FcαRb lacks the transmembrane and intracellular domain, which is replaced by 23 new aa. This is the consequence of alternative splicing that skips the 3-splice site at the end of the EC2 exon, resulting in the insertion of 23 new aa before the stop codon. FcαRb has been described in both neutrophils and eosinophils.38 Although FcαRb can be expressed on the cell membrane—which is probably due to the 23 aa insertion—it is presented mainly as a soluble form. Furthermore, cell-surface-expressed FcαRb is unable to associate with FcR γ-chain and therefore unable to exert cellular signaling after binding to IgA complexes. This suggests that full-length FcαRI and soluble FcαRb on granulocytes may compete for IgA binding. As such, FcαRb may downregulate signaling via full-length FcαRI. A FcαRb alternative spliced transcript (FcαRbΔS2) has been described as well, in which the leader peptide 2 is deleted. However, it is not clear whether this transcript is translated into a functional protein. A second soluble FcαRI has been described that is specifically expressed by monocytes, and which is the result of crosslinking of full-length FcαRI, which triggers FcR γ-chain-dependent shedding.39 Interestingly, release of soluble FcαRI—which is likely due to proteolytic cleavage—is induced by IgA aggregates, suggesting a regulatory effect on FcαRI effector functions. A 2–3 kDa smaller protein variant of full-length FcαRI (with a core protein of 29–30 kDa) was described in neutrophils, as well.40 The nature of this variant is not clear at present.
In addition, mRNA encoding FcαRIa.3 that lacks EC2 has been identified in granulocytes and monocytes.41 Cells, which were transfected with this variant, were able to bind SIgA, but not serum IgA. Furthermore, tumor necrosis factor-α specifically increased or decreased FcαRIa.3 transcripts compared with full-length FcαRIa.1 transcripts in neutrophils or monocytes, respectively.42 Other alternative spliced transcripts have been described on mRNA, but not at protein level, in primary cells or cell lines.34, 37, 41, 42, 43, 44 These include the ΔS2 transcript, in which the leader peptide 2 is deleted, ΔS2EC1, in which both leader peptide 2 and EC1 are eliminated, and transcripts with (part of) EC2 deletions, including ΔS2EC2 (deletion of leader peptide 2 and EC2), ΔS266EC2 (deletion of part leader peptide 2 and 66 bp of EC2), and ΔS274EC2 (missing leader peptide 2 and 74 bp of EC2). Biological significance is not yet completely understood, although different levels of full-length and alternative spliced FcαRI transcripts have been documented in several diseases.30, 42 For example, neutrophils from patients suffering from pneumonia have lower FcαRIa.3 transcripts, whereas monocytes from patients with IgA nephropathy solely express full-length FcαRI. As such, protein isoforms may diversify FcαRI structure and function in immunoregulation of IgA-mediated host defense.
Several single-nucleotide polymorphisms (SNPs) have been described in the FCAR gene as well.29, 31, 45, 46, 47, 48, 49, 50, 51 These constitute three different SNPs within the FCAR gene promotor region, namely −340G/A, −311T/C, and −142T/C, of which the latter two show lower promoter activity within the T allele. Furthermore, two non-coding SNPs (324A/G and 363A/G) are documented within the EC1. The SNP 376G/A results in an aa change from aspartic acid into asparagine. The effect of this variation on IgA binding is not known, although it is located closely to the ligand binding site.52 Finally, a functional SNP has been identified in the intracellular domain (844A/G), which results in an aa transition from serine to glycine (S248–G248).50 IgA-mediated crosslinking of neutrophil FcαRI–G248 triggered significantly more interleukin (IL)-6 release than equivalent crosslinking of the FcαRI–S248 variant. Remarkably, only FcαRI–G248, is capable of inducing cytokine release in the absence of FcR γ-chain, which is presumed due (at least in part) to its ability to interact directly with the Src family member Lyn, an important component of the FcαRI signaling cascade.50
Association of FCAR gene polymorphisms with diseases has been investigated to some extent. Until now, none of the SNPs were associated with allergy,52 but altered susceptibility to aggressive periodontitis (324A/G SNP),48 systemic lupus erythematosus (844A/G SNP),50 and chronic HCV infection (−311T/C and −142T/C SNPs) have been described.49 Furthermore, the (844A/G SNP) influences susceptibility to systemic lupus erythematosus, but not systemic sclerosis or rheumatoid arthritis,50, 53 whereas controversy exists for FcαRI polymorphisms in relation to susceptibility to IgA nephropathy.31, 46, 54
Expression and Modulation
FcαRI expression is already observed at the promyelocyte stage in differentiation, and is restricted to cells of the myeloid lineage, including neutrophils, eosinophils, monocytes, and several macrophage subsets (e.g., alveolar, tonsilar, and splenic, but not small intestine macrophages). FcαRI is furthermore expressed on Kupffer cells and on interstitial, CD34+-derived dendritic cells (DCs) and monocyte-derived DCs (although the latter may be reflected by FcαRI expression on monocytes, which decreases during DC differentiation).34, 35, 36, 37, 55, 56, 57, 58, 59 Expression has recently been described on human platelets as well, whereas FcαRI is not expressed on mast cells or basophils.60 FcαRI expression is constitutive and independent of its ligand, which is demonstrated in IgA-deficient patients who still express FcαRI.61 However, depending on the cell type, expression and function of FcαRI can be modulated by lipopolysaccharide, chemoattractants, inflammatory cytokines, or adapter protein, binding to the intracellular domain of FcαRI.25, 62 For instance, upregulation is induced by IL-1β, tumor necrosis factor-α, granulocyte-macrophage-colony stimulating factor, and IL-8,63, 64, 65, 66 whereas expression is downregulated by transforming growth factor-β, interferon-γ, or the ligand polymeric IgA.67, 68 Upregulation of neutrophil FcαRI expression levels can be the result from either de novo synthesis or transport from intracellular stores to the cell surface.40 Full-length FcαRI is present in both secretory and tertiary granules, whereas the 2–3 kDa smaller variant (core protein of 29–30 kDa) is also present in tertiary granules. As these granules are differently mobilized during neutrophil activation or inflammatory responses, distinct biological functions for FcαRI are suggested, but this has not yet been investigated thoroughly. Tissue distribution of FcαRI is mostly defined by the presence of neutrophils and few emigrated macrophages.69 These cells are evident as clusters in tonsils and appendix, and are scattered in varying numbers in lymph nodes, kidneys, livers, intestinal mucosa, bronchoalveolar lavages, or peritoneal fluid. Inflamed intestines display major influxes of FcαRI-positive neutrophils. The level of FcαRI on neutrophils was similar in tissue compared with blood neutrophils, whereas FcαRI expression on monocytes was much lower in tissues than in blood. Altered FcαRI expression has been reported in diseases such as ankylosing spondylitis, allergic diseases, human immunodeficiency virus (HIV) infection, or bacterial infections.36, 70, 71, 72
Ligand Binding
Although all forms of IgA are ligands for FcαRI, the binding capacities differ. Although monomeric IgA and dimeric IgA are capable of binding FcαRI with moderate affinity (Ka=∼106 M−1) in the boundaries of Cα2 and Cα3, IgA–immune complexes bind avidly.73, 74, 75, 76, 77 Furthermore, because of the partial overlap of the IgA binding site for either FcαRI or pIgR, interaction of SIgA to FcαRI is (partly) hampered because of steric hindrance of SC.75, 78, 79, 80 SIgA binding is however increased when complement receptor 3 functions as coreceptor.
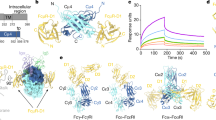
Crystallographic studies demonstrated that one IgA molecule can simultaneously bind two FcαRI molecules (Figure 1).75, 76 This is in contrast to FcγRIII and FcɛRI, for which a 1:1 stoichiometry with their respective ligands was described, again emphasizing dissimilarities between FcαRI and other members of the Fc receptor family.81, 82, 83, 84, 85 Moreover, the IgA binding site on FcαRI is located in EC1 (Figure 1, depicted as yellow aa),75, 76, 79 which is different compared with FcɛRI and FcγRs, as these FcRs bind their ligands in EC2. Residues Y35 (in the BC loop), R52, R53, L54, K55 (in the D strand), F56, W57, N58 (in the DE loop), Y81, R82, I83, G84, H85, and Y86 (in the FG loop) within EC1 are involved in IgA binding.73, 74, 75 Surprisingly, alternative spliced FcαRI, which lacks EC2, does not bind serum IgA, but binds SIgA comparable to full-length FcαRI.41
Schematic representation of the FcαRI–FcR γ-chain complex, binding immunoglobulin A (IgA) in a 2:1 stoichiometry. Two FcαRI bind each IgA-Fc part at the Cα2 and Cα3 junction via extracellular (EC) 1. Amino acids (aa) involved in ligand-receptor binding are depicted in yellow for FcαRI–EC1 and in blue for aa in Cα2 and Cα3 of IgA (left part of figure). Deglycosylation of N58 in FcαRI-EC1 (bold) increases IgA binding. Amino acids in the transmembrane regions (TMs) of FcαRI and a FcR γ-chain homodimer, involved in complex formation, are depicted in red, whereas aa involved in signaling are shown in green. FcαRI intracellular (IC) serine 248 and 263, respectively, modulates FcR γ-chain independent interleukin (IL)-6 production and inside–out signaling. FcR γ-chain intracellular immunoreceptor tyrosine-based activation motif (ITAM) consensus (YxxLx7YxxL) is shown, as well as the disulfide bond between two cysteines at position 7 (solid line). Monoclonal antibodies and C-reactive protein recognizing different ECs of FcαRI are depicted (right part).
Residues within IgA that are involved in FcαRI binding (Figure 1, depicted as blue aa) are L256, L257, and L258 in the α-helix of the AB loop of Cα2. Within Cα3 E348 (A-strand), R382, L384 (C-strand), S387, E389 (CC’ loop), M433, H436 (F-strand), E437, A438, L439, P440, L441, A442 (FG loop), F443, T444, and Q445 (G strand) are involved.75, 86 Immune complexes with optimal binding contain five to six molecules of IgA per complex.87 A number of conformational changes have been observed within the FcαRI–EC1 domain (in the D-strand, DE and FG loop) after binding to IgA, but the approximately 90° orientation of the two EC domains do not change significantly.75 Furthermore, both FcαRI and IgA are heavily glycosylated proteins. FcαRI harbors six N-glycosylation sites and several putative O-linked glycosylation sites, and it has been demonstrated that deglycosylation of FcαRI N58 increases IgA binding.88 By contrast, IgA Fc glycosylation is not critical for binding to FcαRI.88, 89, 90 Several monoclonal Abs (mAbs) have been described that bind FcαRI (Figure 1, right part) in EC1 (My43, 2E6, 2D11, 7G4, 2H8, and MIP8a), EC2 (A59, A77, A62, and 7D7), or have binding sites in both ECs (A3).34, 91, 92 All EC1 recognizing mAbs block IgA binding, whereas mAb A62 recognizes low glycosylated FcαRI. Recently, it was described that the pentraxin C-reactive protein binds to FcαRI, which induces cellular activation. C-reactive protein binds to distinct regions of FcαRI compared with IgA, suggesting simultaneous binding of both molecules to FcαRI.93
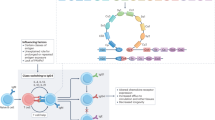
Ligand binding to FcαRI is regulated via a mechanism referred to as inside–out signaling, which entails that cytokine stimulation of cells rapidly, modulates binding capacity in response to intracellular signals, without affecting receptor expression levels.94, 95, 96, 97, 98 Experiments with eosinophils, monocytes, and transfectants demonstrated that FcαRI shows low capacity to interact with IgA–immune complexes in a resting state, but ligand binding capacity increases profoundly after stimulation with cytokines such as granulocyte-macrophage-colony stimulating factor, and IL-4 or IL-5. As such, FcαRI becomes primed, but surface receptor expression is not augmented.96, 97, 98 Inside–out signaling critically depends on the intracellular domain of FcαRI and on the presence of an intact cytoskeleton, but it does not require FcR γ-chain.96, 97 In more detail, phosphorylation of serine 263 (Figure 1, depicted in green), and activation of phosphatidylinositol 3-kinase (PI3K) and its downstream target protein kinase C are essential in switching inactive FcαRI into an active, ligand binding receptor (Figure 2a). Simultaneously, cytokine stimulation induces binding of the serine–threonine phosphatase protein protein phosphatase 2A to the intracellular domain of FcαRI, which results in dephosphorylation of the FcαRI intracellular domain, allowing binding of IgA–immune complexes.98 Whether FcαRI priming is a result of enhanced lateral movement (avidity) and/or conformational changes within the receptor (affinity), as described for integrins,99 is currently unknown. It is not clear whether FcαRI on neutrophils needs priming as well, as activation of neutrophils during isolation has, as of yet, precluded these experiments. However, neutrophils of patients suffering from active, ongoing dermatitis herpetiformis (DH) demonstrate increased ability to bind IgA, without increased receptor expression, which is consistent with a pattern of receptor priming.100 This suggests that priming is the result of ongoing mucosal inflammatory responses and concomitantly systemic cytokine release in patients with dermatitis herpetiformis.
Simplified scheme of signaling pathways involved in FcαRI functioning. (a) Inside–out signaling or priming of FcαRI. The intracellular domain of FcαRI, an intact cytoskeleton, phosphorylation of serine 263, phosphatidylinositol 3 kinase (PI3K), and its downstream target protein kinase C (PKC) and serine/threonine phosphatase protein protein phosphatase 2A (PP2A) are involved in switching inactive FcαRI into an active, ligand binding receptor after cytokine stimulation. (b, Left) Crosslinking of FcαRI by immunoglobulin A (IgA)–immune complexes induces redistribution of FcαRI to plasma membrane rafts. Src kinase Lyn phosphorylates the tyrosines within the associated FcR γ-chain-immunoreceptor tyrosine-based activation motif (ITAM). These then serve as “docking” sites for recruitment of B lymphocyte kinase (Blk), Syk, phospholipase (PLC)-γ, Shc, and growth factor receptor-bound protein 2 (Grb2), which facilitates activation of multiple (and subsequential) targets such as PI3K, PLC-γ, and components of a Grb2 containing multimolecular adapter protein complex. This results in cellular functions such as phagocytosis, Ab-dependent-cellular cytotoxicity, respiratory burst, degranulation, antigen-presentation, and release of cytokines and inflammatory mediators. (Right) Triggering FcαRI with monomeric serum IgA (not crosslinking FcαRI) transduces inhibitory signals through FcαRI–FcR γ-chain complex via inhibitory capacity through FcR γ-chain ITAM (ITAMi), which downregulates other activating Fc receptors. The inhibitory signal involves recruitment of Src homology region 2 domain-containing phosphatase-1 (SHP-1) to FcαRI, and formation of inhibisome clusters (dotted lines), which impair phosphorylation of Syk, LAT, and ERK. Ca2+, calcium; Cbl, Casitas B-lineage lymphoma; DAG, diacylglycerol; GDP, guanosine diphosphate; GTP, guanosine triphosphate; LAT, linker of activated T cells; p, phosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-triphosphate; SLP-76, SH2 domain containing leukocyte protein of 76 kDa; SHIP, Src homology-2-containing inositol 5′-phosphatase.
IgA-Mediated FcαRI Signaling and Cellular Functioning
Binding of IgA–immune complexes (containing either monomeric IgA or dimeric IgA) induces proinflammatory responses, which requires association of FcαRI with the FcR γ-chain subunit.101, 102, 103 Two basic aspects are essential for tethering FcαRI and FcR γ-chain into a stable FcαRI–FcR γ-chain complex. First, dimerization of two γ-chains—through disulfide bond between two cysteines—is required, and second, association between the positively charged arginine 209 (R209) in the TM of FcαRI and an opposite negatively charged aspartic acid 11 (D11) in the TM of FcR γ-chain is essential (Figure 1, depicted in red). Moreover, it was demonstrated that orientation of the positive charge within the TM of FcαRI into the vicinity of the FcR γ-chain dimer is important.104 In addition, two leucines (L14 and L21), two tyrosine (Y17 and Y25), and a cysteine at position 26 (C26), within FcR γ-chain, as well as three leucines on position 217, 220, and 224, within FcαRI TM, contribute to the stabilization of the FcαRI–FcR γ-chain complex105, 106 (Figure 1, depicted in red). In vivo studies in FcαRI transgenic–FcR γ-chain knockout mice demonstrate that FcR γ-chain association is essential for FcαRI surface expression.103 However, FcαRI expression in the absence of FcR γ-chains (FcαRI “γ-less”) was observed in transfected cell lines, and selective human monocyte and neutrophil populations.107, 108, 109 Functionality of FcαRI “γ-less” receptors is limited to ligand binding (inside–out signaling) and receptor recycling via early endosomes, although FcαRI–Gly248 was shown to trigger IL-6 production in the absence of FcR γ-chain.50
The mechanism of FcαRI binding to FcR γ-chain underscores the difference with other FcRs, but shows similarities with members of the LRC family.22, 74 Within the LRC family, an N-terminal positively charged arginine is highly conserved among all activatory receptors, with the exception of killer cell activatory receptors, which bear a positively charged lysine in the center of their TM. Interestingly, killer cell activatory receptors interact with the signaling molecule DAP12, whereas other LRC stimulatory receptors associate (like FcαRI) with the FcR γ-chain.110, 111 In addition, paired Ig-like receptor-A associates with the FcR γ-chain via an transmembrane N-terminal arginine. By contrast, FcR γ-chain association of other activating Fc receptors is based on the residues in the C-terminal part of their TMs.112, 113, 114 For example, FcγRI requires a domain of 10 aa, including an asparagine and FcɛRI, and FcγRIIIa require a C-terminal aspartic acid for FcR γ-chain association.
Crosslinking of FcαRI by IgA–immune complexes induce FcR γ-chain independent redistribution of FcαRI to plasma membrane rafts.115 Furthermore, both the tyrosine kinase Bruton's tyrosine kinase and src family kinase Lyn are recruited to these signaling platforms. FcαRI crosslinking furthermore initiates immunoreceptor tyrosine-based activation motif (ITAM)-dependent signaling of the FcαRI-associated FcR γ-chain (Figure 2b, left part).23, 116 The FcR γ-chain ITAMs consists of a conserved stretch of aa of paired tyrosines and leucines in a consensus sequence (YxxLx7YxxL, Figure 1, depicted in green). Src kinase Lyn phosphorylates the tyrosines within the associated FcR γ-chain–ITAM. These then serve as the “docking” sites for recruitment of other tyrosine kinases, including B lymphocyte kinase and the Src homology 2 (SH2)-domain-containing proteins Syk, phospholipase C-γ, Shc, and growth factor receptor-bound protein 2, which facilitates the activation of multiple targets such as PI3K (with downstream PDK1, protein kinase Cɛ and δ, PKBα, and Bruton's tyrosine kinase activation) and phospholipase C-γ (with downstream release of IP3 and diacylglycerol to trigger calcium release and activation of calcium- and diacylglycerol-dependent protein kinase Cα) (Figure 2b, left part).115, 117, 118, 119, 120 Of note, these pathways are interconnected, for example, Bruton's tyrosine kinase can potentiate calcium signaling—via phospholipase Cγ—but is activated by binding to the PI3K product phosphatidylinositol (3,4,5)-triphosphate via its PH domain. Moreover, Src family kinases, that are activated after FcαRI stimulation, also induces the formation of multimolecular adapter protein complexes consisting of the adapter growth factor receptor-bound protein 2—which is constitutively bound to Sos—and recruited upon phosphorylation of Shc. Furthermore, this complex contains Src homology-2-containing inositol 5′-phosphatase, Casitas B-lineage lymphoma, SH2 domain containing leukocyte protein of 76 kDa, and Crkl.121 Through this adapter complex, GDP–Ras is exchanged to active GTP–Ras by Sos (a guanine nucleotide exchange factor), that is activated by PI3K), which, in turn, activates Raf-1–MEK–MAP serine–threonine kinases by sequential phosphorylation. The interconnected signaling pathways couple upstream FcR γ-chain ITAM phosphorylation to different cellular processes, such as gene expression by activation of several transcription factors (including nuclear factor-κB, AP-1, and Sp1), phagocytosis, Ab-dependent-cellular cytotoxicity, respiratory burst, degranulation, antigen presentation, and release of cytokines and inflammatory lipid mediators.103, 109, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130 Of note, depending on cell type or cell stimulation, FcαRI activation may trigger cell type-specific signaling and functional responses.
Intriguingly, it was demonstrated that non-targeted monomeric serum IgA (not crosslinking FcαRI) transduces inhibitory signals through the FcαRI–FcR γ-chain complex, which downregulates IgE- or IgG-, Fc-receptor-mediated phagocytosis, chemotaxis, bacterial activity, oxidative burst activity, and cytokine release.131, 132, 133, 134, 135, 136, 137, 138, 139 The underlying molecular mechanisms involves ERK-dependent recruitment of tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1 and FcαRI to lipid rafts (Figure 2b, right part).131, 140 In a second step, ligation of the activating Fc receptor results in colocalization with FcαRI and Src homology region 2 domain-containing phosphatase-1 in rafts. These assembled proteins form intracellular structures called “inhibisomes,” which induce impairment of Syk, LAT, and ERK phosphorylation and functionality of activating Fc receptors. Formation of inhibisomes requires Src homology region 2 domain-containing phosphatase-1-dependent depolarization of actin. Sustained aggregation of FcαRI by multimeric ligands by contrast stimulates cell activation by recruiting high amounts of Syk (described above), whereas Src homology region 2 domain-containing phosphatase-1 binding is aborted.131 The inhibitory capacity through FcR γ-chain ITAM is referred to as ITAMi, which differs from the classical immunoreceptor tyrosine-based inhibitory motif signaling of inhibitory Fc receptors, which requires co-aggregation with the activatory Fc receptor.23, 141 Thus, both IgA-induced activating and inhibitory signals depend on FcαRI–FcR γ-chain ITAM, but differ in the recruitment of tyrosine kinases versus tyrosine phosphatases, respectively, (Figure 2b). As such, it has been proposed that crosslinking of FcαRI during infection with IgA-opsonized pathogens results in proinflammatory responses, whereas naturally occurring serum IgA (not complexed with an antigen) induces inhibitory signals through FcαRI to dampen excessive immune responses (initiated by other Ig–immune complexes).
FcαRI and IgA in Mucosal Immunology
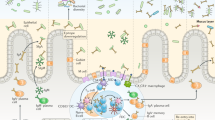
Only few FcαRI-positive cells are observed in mucosal areas in homeostatic conditions. For instance, intestinal macrophages lack FcαRI expression,58 which is consistent with an anti-inflammatory role of IgA to protect mucosal integrity. Furthermore, mucosal Langerhans cells do not express FcαRI. However, low FcαRI levels were observed on in vitro cultured monocyte-derived DCs, which resemble interstitial DC human epithelial interstitial-type DC. A study by Heystek et al.59 demonstrated low level expression of FcαRI on immature monocyte-derived DCs as well. Crosslinking of FcαRI led to internalization of (serum) IgA complexes and antigen presentation through the major histocompatibility complex class II pathway, but not via major histocompatibility compatibility class I cross-presentation, which resulted in monocyte-derived DCs maturation and production of IL-10.57, 120, 142 Because IL-10 mediates IgA isotype switching,143 a role for FcαRI-positive DC in mounting specific immune responses is supported. However, it should be noted that, although FcαRI is able to mediate efficient antigen presentation in FcαRI-expressing transfectants-,109 uptake of IgA immune complexes by DCs, expressing low levels of FcαRI, led to poor antigen presentation.144 Efficient uptake of SIgA was demonstrated, which was partially blocked by anti-mannose receptor mAb, but not by anti-FcαRI blocking mAbs. This indicated that monocyte-derived DCs did not internalize SIgA via FcαRI, but through interaction with carbohydrate-recognizing receptors. Because uptake of SIgA was not accompanied by DC maturation, it was suggested that internalization of SIgA by DC might have a role in maintaining self-tolerance against commensal bacteria. SIgA furthermore has an important role as the first line of defense by preventing penetration of mucosal surfaces by microorganisms or foreign antigens (Figure 3a).1 SIgA inhibits adherence of microorganisms, can agglutinate microbes, and interfere with bacterial motility by interacting with their flagella. Moreover, SIgA neutralizes bacterial products such as enzymes and toxins. However, opsonic activity is poor compared with dIgA or serum IgA as a result of (partial) blockage of the FcαRI binding site by SC, which is consistent with a more anti-inflammatory role of SIgA. By contrast, both dIgA and serum IgA have a dual role in immunity, as they can show both anti- and proinflammatory roles.
Model for the role of FcαRI in mucosal immunity. In humans, immunoglobulin A (IgA) is expressed in three different forms. (a) In homeostatic conditions dimeric IgA (dIgA) functions as intermediary molecule that is secreted as SIgA, which inhibits bacterial invasion. Monomeric serum IgA functions as anti-inflammatory molecule through targeting FcαRI–inhibitory capacity through FcR γ-chain ITAM (ITAMi) signaling. (b) When microorganisms have been able to breach the epithelial barrier, dIgA can opsonize these pathogens. Recruited neutrophils that express FcαRI will clear the infection through phagocytosis and release of leukotrien B4 (LTB4, via active immunoreceptor tyrosine-based activation motif (ITAM) signaling), which may lead to a self-controlled positive feedback loop, until pathogens have been eliminated. Hence, in case of local bacterial translocation, dIgA represents a proinflammatory molecule and functions as the second line of innate mucosal immune defense. (c) Finally, pathogens that have entered the portal circulation are opsonized by serum IgA, and subsequently phagocytosed by FcαRI-positive Kupffer cells. As such, serum IgA can function as proinflammatory antibody when systemic bacterial translocation occurs, and interaction with FcαRI on Kupffer cells represent a third line of defence at the interface of mucosal and systemic immunity. Green, non- or anti-inflammatory and red, proinflammatory.
In homeostatic conditions, dIgA serves as an intermediary that is transported through epithelial cells and released as SIgA. Although antibodies in general have a limited benefit against intracellular pathogens, dIgA can passively neutralize intracellular viruses because of trans-epithelial cell transport by intersecting virus particles and interfering with virus replication or assembly. IgA–virus complexes are subsequently removed via the lumen (Figure 3a). It was demonstrated that addition of specific anti-viral IgA to the basolateral surface of polarized epithelial cells in vitro decreased virus titers of Sendai virus, rotavirus, influenza, or HIV.145, 146, 147, 148, 149 Furthermore, culture with polymeric IgA against toxin A of Clostridium difficile prevented destruction of epithelial monolayers.150 In vivo evidence supporting that IgA transcytosis is required for viral inactivation was obtained when mice were only protected against rotavirus infection when IgA mAbs were given systemically, but not when they were provided via the lumen of the intestinal tract.151 A proinflammatory role for dIgA was recently proposed to eliminate pathogens that have successfully evaded the epithelial barrier. Bacteria, which are opsonized with dIgA, are efficiently phagocytosed by neutrophils.123 Moreover, interaction of dIgA with neutrophil FcαRI leads to release of leukotrien B4, which is a potent neutrophil chemoattractant (Figure 3b).130 As such, it is hypothesized that a self-contained positive feedback loop is initiated, which results in enhanced recruitment of neutrophils, until clearance of invading pathogens has been achieved. Thus, dIgA functions as active second line of defense at mucosal surfaces by recruiting neutrophils.
The function of serum IgA in immunity is even more complicated and incompletely understood. It was demonstrated that IgA has the ability to downregulate IgG-mediated effector functions by transducing inhibitory signals through FcαRI.133, 134, 135, 136, 137, 138, 139 However, Kupffer cells in the liver, which are essential for elimination of bacteria in the portal circulation that have invaded via the gut, were demonstrated to express FcαRI.56 In addition, serum IgA-opsonized Escherichia coli bacteria were efficiently phagocytosed by Kupffer cells, supporting a role for serum IgA in clearance of pathogens at the interface between mucosal and systemic immunity. Thus, crosslinking of FcαRI on Kupffer cells by serum IgA functions as the third line of defense (Figure 3c).
The importance of FcαRI in mucosal infections was supported by two in vivo studies in human FcαRI transgenic mice, which express FcαRI on myeloid cells, similarly as in humans.103, 152 When mice were infected with Bordetella pertussis that had been opsonized with human IgA, enhanced bacterial clearance in lungs of human FcαRI transgenic mice was observed compared with non-transgenic littermates.124 Furthermore, it was recently shown that passive transfer of human IgA mAbs against Mycobacterium tuberculosis protected human FcαRI transgenic mice, but not FcαRI-negative control mice, against M. tuberculosis infection.153 To date, enhanced in vitro uptake of E. coli, Streptococcus pneumonia, Staphylococcus aureus, Porphyromonas gingivalis, Candida albicans, B. pertussis, and Neisseria meningitidis by neutrophils after targeting of FcαRI have been demonstrated.56, 123, 124, 130, 154, 155, 156
FcαRI as Therapeutic Target
Because FcαRI has a dual role in immunity, as naturally occurring serum IgA induces inhibitory signals to dampen excessive immune responses, whereas crosslinking of FcαRI during infection with IgA-opsonized pathogens results in proinflammatory responses; manipulation of FcαRI function may offer novel promising therapeutic strategies. Until now, no (bispecific) Ab (BsAb)-based therapies that target FcαRI exist in the clinic. Most of the research to investigate the potential of IgA mAbs for immunotherapeutical approaches has been based on in vitro experiments, but the increasing availability of suitable mouse models will greatly facilitate future in vivo studies. Two human FcαRI transgenic mouse models are currently available, in which FcαRI is either expressed on monocytes/macrophages or preferentially on neutrophilic granulocytes.103, 157 The complementary FcαRI expression in these models will allow studying the involvement of either cell type in disease or therapeutic efficacy.
Although it has been a challenge in the past to generate sufficient amounts of purified human IgA, technologies to re-clone IgG mAbs, phage display, or transgenic plant technology has resulted in the generation of specific human IgA mAbs (as generation of sufficient BsAb is labor intensive with low yield, and as such may be unrealistic for clinical use). Moreover, the development of human IgA knock-in mouse model useable for standard hybridoma technology,158 and new approaches in IgA purification techniques159, 160, 161 will furthermore allow the production of a continuous source of antigen-specific human IgA.
FcαRI as Anti-Inflammatory Therapeutic Tool
Two experimental inflammatory disease models have been described that demonstrate the potential of dampening excessive immune responses (initiated by other Ig–immune complexes) by inducing ITAMi signaling (Figure 2b) through FcαRI. First, FcαRI transgenic mice (expressing FcαRI on monocytes/macrophages) that were immunized with IgE–immune complexes developed bronchial hyper-reactivity after challenge with the antigen. Treatment with anti-FcαRI Fab, which targets FcαRI monovalently, hereby inducing ITAMi signaling—significantly reduced peribronchial inflammatory cell infiltration as well as symptoms.131 Second, both, decreased inflammatory cell infiltrates and fibrosis were observed in FcαRI mice that were treated with anti-FcαRI Fab in kidney inflammation models.162 Moreover, anti-FcαRI Fabs were shown to induce apoptosis in FcαRI-expressing mast cell transfectants, which prevented tumor development and halted the growth of established tumors.163 As such, targeting ITAMi via FcαRI can initiate either inhibitory signals or apoptosis, which may help to control disproportionate inflammation or tumor development. The therapeutic potential of targeting ITAMi has recently been reviewed by Monteiro et al.141, 164
Targeting FcαRI for Treatment of Infectious Diseases and Cancer
Because IgA is predominantly present in mucosal areas where it shows several important functions (see above), therapies that aim to increase specific IgA titers against mucosal pathogens may help to fight (mucosal) infection. For instance, treatment with specific IgA protected mice against rotavirus, which is a diarrhea-causing pathogen.151 Interestingly, protective effect was only observed when IgA mAbs were given systemically, but not when IgA was presented via the lumen of the intestinal tract. These results therefore not only support the hypothesis that IgA transcytosis is required for intracellular viral inactivation but also suggest that transport of systemically delivered IgA via the pIgR route is not hampered by locally produced mucosal IgA.151 Similarly, increased presence of mucosal IgA by either passive transfer with specific IgA or through oral immunization prevented Helicobacter felis,165 Helicobacter pylori,166 influenza,167, 168 or Shigella flexneri169 infection. Importantly, as these experiments were performed in mice, which lack FcαRI, the protective effect of IgA is presumably even more pronounced in humans. Mucosal administration of an HIV-1 vaccine resulted in resistance to the virus and production of virus-specific IgA with HIV-1 transcystosis-blocking properties in Macaca mulatta monkeys.170 However, it was recently demonstrated that targeting FcαRI directed neutrophils to destroy HIV-infected target cells.171 Because M. mulatta monkeys express FcαRI, an active role for FcαRI in eliciting protection is suggested, in addition to HIV neutralizing IgA Ab. Treatment of human FcαRI transgenic mice with specific IgA induced enhanced protection against B. pertussis or M. tuberculosis infection, compared with non-transgenic littermates.124, 153
FcαRI was furthermore proposed as a novel trigger molecule for mAb-based anti-cancer therapy.172, 173 Although low expression of FcαRI on DCs, poor ability of efficient Ag presentation, and no cross-presentation limits FcαRI targeting on DCs for development of cancer vaccines, it was demonstrated that targeting FcαRI efficiently recruits neutrophils as effector cells. In vitro experiments using therapeutic IgA1, IgA2, dIgA, chimeric IgA, or FcαRI BsAb have provided promising results.127, 128, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186 For instance, neutrophils killed tumor cells much more effectively in the presence of anti-(HER2/neu × FcαRI) BsAb, or anti-EpCAM IgA mAbs compared with an IgG counterpart. Similar superior ability of FcαRI to induce neutrophil-mediated tumor cell killing has now also been demonstrated for epidermal growth factor receptor, human leukocyte antigen class II, CD20, CD30, and carcinoembryonic antigen.127, 172, 173, 174, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186 One explanation for increased Ab-dependent-cellular cytotoxicity after targeting with IgA mAb or FcαRI BsAb may be the induction of neutrophil migration in the presence of IgA. Neutrophil accumulation and destruction of either mamma carcinoma or colon carcinoma colonies in a three-dimensional culture system was only observed when FcαRI was targeted, which is likely the result of leukotrien B4 release after crosslinking of FcαRI.127, 130, 187 However, it was previously shown that immature bone marrow neutrophils were not capable of killing tumor cells via FcγRI, whereas FcαRI efficiently induced Ab-dependent-cellular cytotoxicity.128 It is therefore likely that the amplitude of signals mediated through FcαRI or FcγR also differs, as interaction of FcαRI with FcR γ-chain is stronger because of an electrostatic interaction (Figure 2) that is absent for FcγR.102 An attractive feature of recruiting neutrophils as effector cells is the fact that targeting FcαRI on neutrophils was recently demonstrated to induce autophagic tumor cell death (and necrosis to a lesser extend).188 As such, neutrophils may be able to kill tumor cells with mutations in apoptotic pathways. Moreover, neutrophils attract Th17 cells, which have been shown to have a role in anti-tumor immunity,189, 190 and moreover secrete cytokines and chemokines that attract other immune cells (monocytes or DCs), which may result in more generalized anti-tumor immune responses.191
Conclusion
FcαRI has a significant role in vivo for maintaining appropriate immune responses in both systemic and mucosal compartments. On one hand, FcαRI is involved in the prevention of superfluous immune responses that are initiated through other activating receptors. On the other hand, IgA can potently trigger protective immunity by crosslinking FcαRI on myeloid immune cells. The lack of FcαRI expression in mice has previously seriously hampered in vivo experiments to further elucidate the involvement of FcαRI in anti- and proinflammatory functions. Currently increasing availability of suitable genetically engineered mouse models will significantly facilitate future in vivo studies to establish the complex role of FcαRI in (mucosal) immunity and its potential as therapeutic target for human diseases.
References
Fagarasan, S. & Honjo, T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol 3, 63–72 (2003).
Pamer, E.G. Immune responses to commensal and environmental microbes. Nat. Immunol. 8, 1173–1178 (2007).
Macpherson, A.J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Woof, J.M. & Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 208, 270–282 (2006).
Johansen, F.E., Braathen, R. & Brandtzaeg, P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J. Immunol. 167, 5185–5192 (2001).
Braathen, R., Sorensen, V., Brandtzaeg, P., Sandlie, I. & Johansen, F.E. The carboxyl-terminal domains of IgA and IgM direct isotype-specific polymerization and interaction with the polymeric immunoglobulin receptor. J. Biol. Chem 277, 42755–42762 (2002).
Lewis, M.J., Pleass, R.J., Batten, M.R., Atkin, J.D. & Woof, J.M. Structural requirements for the interaction of human IgA with the human polymeric Ig receptor. J. Immunol. 175, 6694–6701 (2005).
Mostov, K.E. Transepithelial transport of immunoglobulins. Annu. Rev. Immunol. 12, 63–84 (1994).
Shibuya, A. et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1, 441–446 (2000).
Stockert, R.J., Kressner, M.S., Collins, J.C., Sternlieb, I. & Morell, A.G. IgA interaction with the asialoglycoprotein receptor. Proc. Natl Acad. Sci. USA 79, 6229–6231 (1982).
Moura, I.C. et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J. Exp. Med. 194, 417–425 (2001).
Lamkhioued, B. et al. Human eosinophils express a receptor for secretory component. Role in secretory IgA-dependent activation. Eur. J. Immunol. 25, 117–125 (1995).
Mantis, N.J. et al. Selective adherence of IgA to murine Peyer′s patch M cells: evidence for a novel IgA receptor. J. Immunol. 169, 1844–1851 (2002).
Reljic, R. In search of the elusive mouse macrophage Fc-alpha receptor. Immunol. Lett. 107, 80–81 (2006).
Woof, J.M. & Kerr, M.A. IgA function—variations on a theme. Immunology 113, 175–177 (2004).
Maruoka, T., Nagata, T. & Kasahara, M. Identification of the rat IgA Fc receptor encoded in the leukocyte receptor complex. Immunogenetics 55, 712–716 (2004).
Morton, H.C. et al. Cloning and characterization of an immunoglobulin A Fc receptor from cattle. Immunology 111, 204–211 (2004).
Morton, H.C., Pleass, R.J., Storset, A.K., Brandtzaeg, P. & Woof, J.M. Cloning and characterization of equine CD89 and identification of the CD89 gene in chimpanzees and Rhesus macaques. Immunology 115, 74–84 (2005).
Morton, H.C. IgA Fc receptors in cattle and horses. Vet. Immunol. Immunopathol. 108, 139–143 (2005).
Kazeeva, T.N. & Shevelev, A.B. IgA-specific proteins of pathogenic bacteria. Biochemistry (Mosc) 74, 12–21 (2009).
Kremer, E.J. et al. The gene for the human IgA Fc receptor maps to 19q13.4. Hum. Genet. 89, 107–108 (1992).
Martin, A.M., Kulski, J.K., Witt, C., Pontarotti, P. & Christiansen, F.T. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol. 23, 81–88 (2002).
Daeron, M. Fc receptor biology. Annu. Rev. Immunol. 15, 203–234 (1997).
Nimmerjahn, F. & Ravetch, J.V. Fc gamma receptors: old friends and new family members. Immunity 24, 19–28 (2006).
Monteiro, R.C. & Van De Winkel, J.G. IgA Fc receptors. Annu. Rev. Immunol. 21, 177–204 (2003).
Ono, M., Yuasa, T., Ra, C. & Takai, T. Stimulatory function of paired immunoglobulin-like receptor-A in mast cell line by associating with subunits common to Fc receptors. J. Biol. Chem. 274, 30288–30296 (1999).
Maliszewski, C.R., March, C.J., Schoenborn, M.A., Gimpel, S. & Shen, L. Expression cloning of a human Fc receptor for IgA. J. Exp. Med. 172, 1665–1672 (1990).
de Wit, T.P., Morton, H.C., Capel, P.J. & van de Winkel, J.G. Structure of the gene for the human myeloid IgA Fc receptor (CD89). J. Immunol. 155, 1203–1209 (1995).
Morton, H.C., van Egmond, M. & van de Winkel, J.G. Structure and function of human IgA Fc receptors (Fc alpha R). Crit. Rev. Immunol. 16, 423–440 (1996).
Toyabe, S., Kuwano, Y., Takeda, K., Uchiyama, M. & Abo, T. IgA nephropathy-specific expression of the IgA Fc receptors (CD89) on blood phagocytic cells. Clin. Exp. Immunol. 110, 226–232 (1997).
Shimokawa, T., Tsuge, T., Okumura, K. & Ra, C. Identification and characterization of the promoter for the gene encoding the human myeloid IgA Fc receptor (Fc alphaR, CD89). Immunogenetics 51, 945–954 (2000).
Shimokawa, T. & Ra, C. C/EBP alpha and Ets protein family members regulate the human myeloid IgA Fc receptor (Fc alpha R, CD89) promoter. J. Immunol. 170, 2564–2572 (2003).
Shimokawa, T. & Ra, C. C/EBPalpha functionally and physically interacts with GABP to activate the human myeloid IgA Fc receptor (Fc alphaR, CD89) gene promoter. Blood 106, 2534–2542 (2005).
Monteiro, R.C., Kubagawa, H. & Cooper, M.D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J. Exp. Med. 171, 597–613 (1990).
Monteiro, R.C., Cooper, M.D. & Kubagawa, H. Molecular heterogeneity of Fc alpha receptors detected by receptor-specific monoclonal antibodies. J. Immunol. 148, 1764–1770 (1992).
Monteiro, R.C. et al. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J. Clin. Invest. 92, 1681–1685 (1993).
Patry, C., Sibille, Y., Lehuen, A. & Monteiro, R.C. Identification of Fc alpha receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J. Immunol. 156, 4442–4448 (1996).
van Dijk, T.B. et al. Cloning and characterization of Fc alpha Rb, a novel Fc alpha receptor (CD89) isoform expressed in eosinophils and neutrophils. Blood 88, 4229–4238 (1996).
van Zandbergen, G. et al. Crosslinking of the human Fc receptor for IgA (Fc alphaRI/CD89) triggers FcR gamma-chain-dependent shedding of soluble CD89. J. Immunol. 163, 5806–5812 (1999).
Yin, N., Peng, M., Xing, Y. & Zhang, W. Intracellular pools of Fc alphaR (CD89) in human neutrophils are localized in tertiary granules and secretory vesicles, and two Fc alphaR isoforms are found in tertiary granules. J. Leukoc. Biol. 82, 551–558 (2007).
Pleass, R.J., Andrews, P.D., Kerr, M.A. & Woof, J.M. Alternative splicing of the human IgA Fc receptor CD89 in neutrophils and eosinophils. Biochem. J. 318 (Pt 3), 771–777 (1996).
Togo, S., Shimokawa, T., Fukuchi, Y. & Ra, C. Alternative splicing of myeloid IgA Fc receptor (Fc alpha R, CD89) transcripts in inflammatory responses. FEBS Lett. 535, 205–209 (2003).
Reterink, T.J., Verweij, C.L., van Es, L.A. & Daha, M.R. Alternative splicing of IgA Fc receptor (CD89) transcripts. Gene 175, 279–280 (1996).
Pleass, R.J., Dunlop, J.I. & Woof, J.M. Multiple transcripts of human IgA Fc receptor CD89 in neutrophils, eosinophils and the monocyte-like cell line THP-1. Biochem. Soc. Trans. 25, 327S (1997).
van Vuuren, A.J., van Egmond, M., Coenen, M.J., Morton, H.C. & van de Winkel, J.G. Characterization of the human myeloid IgA Fc receptor I (CD89) gene in a cosmid clone. Immunogenetics 49, 586–589 (1999).
Tsuge, T., Shimokawa, T., Horikoshi, S., Tomino, Y. & Ra, C. Polymorphism in promoter region of Fc alpha receptor gene in patients with IgA nephropathy. Hum. Genet. 108, 128–133 (2001).
Jasek, M. et al. A novel polymorphism in the cytoplasmic region of the human immunoglobulin A Fc receptor gene. Eur. J. Immunogenet. 31, 59–62 (2004).
Kaneko, S. et al. A novel polymorphism of Fc alphaRI (CD89) associated with aggressive periodontitis. Tissue Antigens 63, 572–577 (2004).
Watanabe, A. et al. Genetic variants of the IgA Fc receptor (Fc alphaR, CD89) promoter in chronic hepatitis C patients. Immunogenetics 58, 937–946 (2006).
Wu, J. et al. Fc alphaRI (CD89) alleles determine the proinflammatory potential of serum IgA. J. Immunol. 178, 3973–3982 (2007).
Bournazos, S., Woof, J.M., Hart, S.P. & Dransfield, I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin. Exp. Immunol. 157, 244–254 (2009).
Jasek, M. et al. Are single nucleotide polymorphisms of the immunoglobulin A Fc receptor gene associated with allergic asthma? Int. Arch. Allergy Immunol. 135, 325–331 (2004).
Broen, J.C. et al. The functional polymorphism 844 A>G in Fc alphaRI (CD89) does not contribute to systemic sclerosis or rheumatoid arthritis susceptibility. J. Rheumatol. 38, 446–449.
Narita, I. et al. Genetic polymorphisms in the promoter and 5′ UTR region of the Fc alpha receptor (CD89) are not associated with a risk of IgA nephropathy. J. Hum. Genet. 46, 694–698 (2001).
Sibille, Y., Chatelain, B., Staquet, P., Delacroix, D.L. & Vaerman, J.P. IgA receptors on human alveolar macrophages. Monogr. Allergy 24, 282–286 (1988).
van Egmond, M. et al. Fc alphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat. Med. 6, 680–685 (2000).
Geissmann, F. et al. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J. Immunol. 166, 346–352 (2001).
Smith, P.D. et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 167, 2651–2656 (2001).
Heystek, H.C., Moulon, C., Woltman, A.M., Garonne, P. & van Kooten, C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J. Immunol. 168, 102–107 (2002).
Qian, K. et al. Functional expression of IgA receptor Fc alphaRI on human platelets. J. Leukoc. Biol. 84, 1492–1500 (2008).
Chevailler, A., Monteiro, R.C., Kubagawa, H. & Cooper, M.D. Immunofluorescence analysis of IgA binding by human mononuclear cells in blood and lymphoid tissue. J. Immunol. 142, 2244–2249 (1989).
Bakema, J.E. et al. c-Jun activating binding protein 1 binds to the IgA receptor and modulates protein levels of Fc alphaRI and FcR gamma-chain. Eur. J. Immunol. 40, 2035–2040 (2010).
Shen, L., Collins, J.E., Schoenborn, M.A. & Maliszewski, C.R. Lipopolysaccharide and cytokine augmentation of human monocyte IgA receptor expression and function. J. Immunol. 152, 4080–4086 (1994).
Gessl, A. et al. Influence of tumour necrosis factor-alpha on the expression of Fc IgG and IgA receptors, and other markers by cultured human blood monocytes and U937 cells. Scand. J. Immunol. 39, 151–156 (1994).
Hostoffer, R.W., Krukovets, I. & Berger, M. Increased Fc alpha R expression and IgA-mediated function on neutrophils induced by chemoattractants. J. Immunol. 150, 4532–4540 (1993).
Hostoffer, R.W., Krukovets, I. & Berger, M. Enhancement by tumor necrosis factor-alpha of Fc alpha receptor expression and IgA-mediated superoxide generation and killing of Pseudomonas aeruginosa by polymorphonuclear leukocytes. J. Infect. Dis. 170, 82–87 (1994).
Reterink, T.J., Levarht, E.W., Klar-Mohamad, N., Van Es, L.A. & Daha, M.R. Transforming growth factor-beta 1 (TGF-beta 1) down-regulates IgA Fc-receptor (CD89) expression on human monocytes. Clin. Exp. Immunol. 103, 161–166 (1996).
Grossetete, B. et al. Down-regulation of Fc alpha receptors on blood cells of IgA nephropathy patients: evidence for a negative regulatory role of serum IgA. Kidney Int. 53, 1321–1335 (1998).
Hamre, R., Farstad, I.N., Brandtzaeg, P. & Morton, H.C. Expression and modulation of the human immunoglobulin A Fc receptor (CD89) and the FcR gamma chain on myeloid cells in blood and tissue. Scand. J. Immunol. 57, 506–516 (2003).
Grossetete, B., Viard, J.P., Lehuen, A., Bach, J.F. & Monteiro, R.C. Impaired Fc alpha receptor expression is linked to increased immunoglobulin A levels and disease progression in HIV-1-infected patients. AIDS 9, 229–234 (1995).
Chiamolera, M. et al. Enhanced expression of Fc alpha receptor I on blood phagocytes of patients with gram-negative bacteremia is associated with tyrosine phosphorylation of the FcR-gamma subunit. Shock 16, 344–348 (2001).
Montenegro, V., Chiamolera, M., Launay, P., Goncalves, C.R. & Monteiro, R.C. Impaired expression of IgA Fc receptors (CD89) by blood phagocytic cells in ankylosing spondylitis. J. Rheumatol. 27, 411–417 (2000).
Wines, B.D. et al. Identification of residues in the first domain of human Fc alpha receptor essential for interaction with IgA. J. Immunol. 162, 2146–2153 (1999).
Wines, B.D., Sardjono, C.T., Trist, H.H., Lay, C.S. & Hogarth, P.M. The interaction of Fc alpha RI with IgA and its implications for ligand binding by immunoreceptors of the leukocyte receptor cluster. J. Immunol. 166, 1781–1789 (2001).
Herr, A.B., Ballister, E.R. & Bjorkman, P.J. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature 423, 614–620 (2003).
Ding, Y. et al. Crystal structure of the ectodomain of human Fc alphaRI. J. Biol. Chem. 278, 27966–27970 (2003).
Oortwijn, B.D. et al. Monomeric and polymeric IgA show a similar association with the myeloid Fc alphaRI/CD89. Mol. Immunol. 44, 966–973 (2007).
van Spriel, A.B. et al. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 97, 2478–2486 (2001).
Woof, J.M. & Burton, D.R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 4, 89–99 (2004).
Bonner, A., Almogren, A., Furtado, P.B., Kerr, M.A. & Perkins, S.J. Location of secretory component on the Fc edge of dimeric IgA1 reveals insight into the role of secretory IgA1 in mucosal immunity. Mucosal. Immunol. 2, 74–84 (2009).
Shi, J. et al. Interaction of the low-affinity receptor CD23/Fc epsilon RII lectin domain with the Fc epsilon3-4 fragment of human immunoglobulin E. Biochemistry 36, 2112–2122 (1997).
Keown, M.B., Ghirlando, R., Mackay, G.A., Sutton, B.J. & Gould, H.J. Basis of the 1:1 stoichiometry of the high affinity receptor Fc epsilon RI-IgE complex. Eur. Biophys. J. 25, 471–476 (1997).
Sondermann, P., Huber, R., Oosthuizen, V. & Jacob, U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 406, 267–273 (2000).
Keown, M.B., Henry, A.J., Ghirlando, R., Sutton, B.J. & Gould, H.J. Thermodynamics of the interaction of human immunoglobulin E with its high-affinity receptor Fc epsilon RI. Biochemistry 37, 8863–8869 (1998).
Zhang, Y. et al. Crystal structure of the extracellular domain of a human Fc gamma RIII. Immunity 13, 387–395 (2000).
Pleass, R.J., Dunlop, J.I., Anderson, C.M. & Woof, J.M. Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human Fc alpha receptor (Fc alphaR) CD89. J. Biol. Chem. 274, 23508–23514 (1999).
Reterink, T.J. et al. Size-dependent effect of IgA on the IgA Fc receptor (CD89). Eur. J. Immunol. 27, 2219–2224 (1997).
Xue, J., Zhao, Q., Zhu, L. & Zhang, W. Deglycosylation of Fc alphaR at N58 increases its binding to IgA. Glycobiology 20, 905–915 (2010).
Gomes, M.M. et al. Analysis of IgA1 N-glycosylation and its contribution to Fc alphaRI binding. Biochemistry 47, 11285–11299 (2008).
Mattu, T.S. et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J. Biol. Chem. 273, 2260–2272 (1998).
Shen, L., Lasser, R. & Fanger, M.W. My 43, a monoclonal antibody that reacts with human myeloid cells inhibits monocyte IgA binding and triggers function. J. Immunol. 143, 4117–4122 (1989).
Morton, H.C. et al. Immunoglobulin-binding sites of human Fc alphaRI (CD89) and bovine Fc gamma2R are located in their membrane-distal extracellular domains. J. Exp. Med. 189, 1715–1722 (1999).
Lu, J. et al. Recognition and functional activation of the human IgA receptor (Fc{alpha}RI) by C-reactive protein. Proc. Natl Acad. Sci. USA 108, 4974–4979 (2011).
Weisbart, R.H., Kacena, A., Schuh, A. & Golde, D.W. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature 332, 647–648 (1988).
Baldwin, G.C. et al. Granulocyte-macrophage colony-stimulating factor enhances neutrophil function in acquired immunodeficiency syndrome patients. Proc. Natl Acad. Sci. USA 85, 2763–2766 (1988).
Bracke, M. et al. Differential effects of the T helper cell type 2-derived cytokines IL-4 and IL-5 on ligand binding to IgG and IgA receptors expressed by human eosinophils. J. Immunol. 159, 1459–1465 (1997).
Bracke, M., Lammers, J.W., Coffer, P.J. & Koenderman, L. Cytokine-induced inside-out activation of Fc alphaR (CD89) is mediated by a single serine residue (S263) in the intracellular domain of the receptor. Blood 97, 3478–3483 (2001).
Bakema, J.E. et al. Inside-out regulation of Fc alpha RI (CD89) depends on PP2A. J. Immunol. 181, 4080–4088 (2008).
Ginsberg, M.H., Partridge, A. & Shattil, S.J. Integrin regulation. Curr. Opin. Cell Biol. 17, 509–516 (2005).
Smith, A.D., Streilein, R.D. & Hall, R.P. III Neutrophil CD11b, L-selectin and Fc IgA receptors in patients with dermatitis herpetiformis. Br. J. Dermatol. 147, 1109–1117 (2002).
Pfefferkorn, L.C. & Yeaman, G.R. Association of IgA-Fc receptors (Fc alpha R) with Fc epsilon RI gamma 2 subunits in U937 cells. Aggregation induces the tyrosine phosphorylation of gamma 2. J. Immunol. 153, 3228–3236 (1994).
Morton, H.C. et al. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR gamma chain. Molecular basis for CD89/FcR gamma chain association. J. Biol. Chem. 270, 29781–29787 (1995).
van Egmond, M. et al. Human immunoglobulin A receptor (Fc alphaRI, CD89) function in transgenic mice requires both FcR gamma chain and CR3 (CD11b/CD18). Blood 93, 4387–4394 (1999).
Bakema, J.E. et al. Signaling through mutants of the IgA receptor CD89 and consequences for Fc receptor gamma-chain interaction. J. Immunol. 176, 3603–3610 (2006).
Wines, B.D., Trist, H.M., Monteiro, R.C., Van Kooten, C. & Hogarth, P.M. Fc receptor gamma chain residues at the interface of the cytoplasmic and transmembrane domains affect association with Fc alphaRI, surface expression, and function. J. Biol. Chem. 279, 26339–26345 (2004).
Wines, B.D., Trist, H.M., Ramsland, P.A. & Hogarth, P.M. A common site of the Fc receptor gamma subunit interacts with the unrelated immunoreceptors Fc alphaRI and Fc epsilonRI. J. Biol. Chem. 281, 17108–17113 (2006).
Launay, P. et al. Alternative endocytic pathway for immunoglobulin A Fc receptors (CD89) depends on the lack of FcRgamma association and protects against degradation of bound ligand. J. Biol. Chem. 274, 7216–7225 (1999).
Honorio-Franca, A.C., Launay,p P., Carneiro-Sampaio, M.M. & Monteiro, R.C. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J. Leukoc. Biol. 69, 289–296 (2001).
Shen, L. et al. Presentation of ovalbumin internalized via the immunoglobulin-A Fc receptor is enhanced through Fc receptor gamma-chain signaling. Blood 97, 205–213 (2001).
Lanier, L.L., Corliss, B.C., Wu, J., Leong, C. & Phillips, J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998).
Westgaard, I.H. et al. Rat NKp46 activates natural killer cell cytotoxicity and is associated with Fc epsilon RI gamma and CD3zeta. J. Leukoc. Biol. 76, 1200–1206 (2004).
Kim, M.K. et al. Fc gamma receptor transmembrane domains: role in cell surface expression, gamma chain interaction, and phagocytosis. Blood 101, 4479–4484 (2003).
Ravetch, J.V. & Kinet, J.P. Fc receptors. Annu. Rev. Immunol. 9, 457–492 (1991).
Kinet, J.P. The high-affinity receptor for IgE. Curr. Opin. Immunol. 2, 499–505 (1989).
Lang, M.L., Shen, L. & Wade, W.F. Gamma-chain dependent recruitment of tyrosine kinases to membrane rafts by the human IgA receptor Fc alpha R. J. Immunol. 163, 5391–5398 (1999).
Gulle, H., Samstag, A., Eibl, M.M. & Wolf, H.M. Physical and functional association of Fc alpha R with protein tyrosine kinase Lyn. Blood 91, 383–391 (1998).
Launay, P., Lehuen, A., Kawakami, T., Blank, U. & Monteiro, R.C. IgA Fc receptor (CD89) activation enables coupling to syk and Btk tyrosine kinase pathways: differential signaling after IFN-gamma or phorbol ester stimulation. J. Leukoc. Biol. 63, 636–642 (1998).
Shen, L., Lang, M.L. & Wade, W.F. The ins and outs of getting in: structures and signals that enhance BCR or Fc receptor-mediated antigen presentation. Immunopharmacology 49, 227–240 (2000).
Lang, M.L. et al. Fc alpha receptor cross-linking causes translocation of phosphatidylinositol-dependent protein kinase 1 and protein kinase B alpha to MHC class II peptide-loading-like compartments. J. Immunol. 166, 5585–5593 (2001).
Chen, Y.W., Lang, M.L. & Wade, W.F. Protein kinase C-alpha and -delta are required for Fc alphaR (CD89) trafficking to MHC class II compartments and Fc alphaR-mediated antigen presentation. Traffic 5, 577–594 (2004).
Park, R.K., Izadi, K.D., Deo, Y.M. & Durden, D.L. Role of Src in the modulation of multiple adaptor proteins in Fc alphaRI oxidant signaling. Blood 94, 2112–2120 (1999).
Lang, M.L. & Kerr, M.A. Characterization of Fc alphaR-triggered Ca(2+) signals: role in neutrophil NADPH oxidase activation. Biochem. Biophys. Res. Commun. 276, 749–755 (2000).
Vidarsson, G. et al. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J. Immunol. 166, 6250–6256 (2001).
Hellwig, S.M., van Spriel, A.B., Schellekens, J.F., Mooi, F.R. & van de Winkel, J.G. Immunoglobulin A-mediated protection against Bordetella pertussis infection. Infect. Immun. 69, 4846–4850 (2001).
Ouadrhiri, Y., Pilette, C., Monteiro, R.C., Vaerman, J.P. & Sibille, Y. Effect of IgA on respiratory burst and cytokine release by human alveolar macrophages: role of ERK1/2 mitogen-activated protein kinases and NF-kappaB. Am. J. Respir. Cell Mol. Biol. 26, 315–332 (2002).
Pilette, C., Detry, B., Guisset, A., Gabriels, J. & Sibille, Y. Induction of interleukin-10 expression through Fc alpha receptor in human monocytes and monocyte-derived dendritic cells: role of p38 MAPKinase. Immunol. Cell Biol. 88, 486–493.
Otten, M.A. et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J. Immunol. 174, 5472–5480 (2005).
Otten, M.A. et al. FcR gamma-chain dependent signaling in immature neutrophils is mediated by Fc alphaRI, but not by Fc gammaRI. J. Immunol. 179, 2918–2924 (2007).
Pleass, R.J., Lang, M.L., Kerr, M.A. & Woof, J.M. IgA is a more potent inducer of NADPH oxidase activation and degranulation in blood eosinophils than IgE. Mol. Immunol. 44, 1401–1408 (2007).
van der Steen, L. et al. Immunoglobulin A: Fc(alpha)RI interactions induce neutrophil migration through release of leukotriene B4. Gastroenterology 137, 2018–2029 e2011–2013 (2009).
Pasquier, B. et al. Identification of Fc alphaRI as an inhibitory receptor that controls inflammation: dual role of FcR gamma ITAM. Immunity 22, 31–42 (2005).
Kanamaru, Y., Blank, U. & Monteiro, R.C. IgA Fc receptor I is a molecular switch that determines IgA activating or inhibitory functions. Contrib. Nephrol. 157, 148–152 (2007).
Van Epps, D.E. & Brown, S.L. Inhibition of formylmethionyl-leucyl-phenylalanine-stimulated neutrophil chemiluminescence by human immunoglobulin A paraproteins. Infect. Immun. 34, 864–870 (1981).
Van Epps, D.E. & Williams, R.C., Jr . Suppression of leukocyte chemotaxis by human IgA myeloma components. J. Exp. Med. 144, 1227–1242 (1976).
Van Epps, D.E., Reed, K. & Williams, R.C., Jr . Suppression of human PMN bactericidal activity by human IgA paraproteins. Cell Immunol. 36, 363–376 (1978).
Wilton, J.M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin. Exp. Immunol. 34, 423–428 (1978).
Wolf, H.M. et al. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 83, 1278–1288 (1994).
Wolf, H.M. & Eibl, M.M. The anti-inflammatory effect of an oral immunoglobulin (IgA-IgG) preparation and its possible relevance for the prevention of necrotizing enterocolitis. Acta. Paediatr. Suppl. 396, 37–40 (1994).
Nikolova, E.B. & Russell, M.W. Dual function of human IgA antibodies: inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J. Leukoc. Biol. 57, 875–882 (1995).
Pfirsch-Maisonnas, S. et al. Inhibitory ITAM signaling traps activating receptors with the phosphatase SHP-1 to form polarized “inhibisome” clusters. Sci. Signal. 4, ra24 (2011).
Blank, U., Launay, P., Benhamou, M. & Monteiro, R.C. Inhibitory ITAMs as novel regulators of immunity. Immunol. Rev. 232, 59–71 (2009).
Pasquier, B., Lepelletier, Y., Baude, C., Hermine, O. & Monteiro, R.C. Differential expression and function of IgA receptors (CD89 and CD71) during maturation of dendritic cells. J. Leukoc. Biol. 76, 1134–1141 (2004).
Fayette, J. et al. Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2. J. Exp. Med. 185, 1909–1918 (1997).
Otten, M.A., Groenveld, I., van de Winkel, J.G. & van Egmond, M. Inefficient antigen presentation via the IgA Fc receptor (Fc alphaRI) on dendritic cells. Immunobiology 211, 503–510 (2006).
Mazanec, M.B., Kaetzel, C.S., Lamm, M.E., Fletcher, D. & Nedrud, J.G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl Acad. Sci. USA 89, 6901–6905 (1992).
Mazanec, M.B., Coudret, C.L. & Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69, 1339–1343 (1995).
Mazanec, M.B. et al. Intracellular neutralization of Sendai and influenza viruses by IgA monoclonal antibodies. Adv. Exp. Med. Biol. 371A, 651–654 (1995).
Devito, C. et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165, 5170–5176 (2000).
Corthesy, B. et al. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 80, 10692–10699 (2006).
Stubbe, H., Berdoz, J., Kraehenbuhl, J.P. & Corthesy, B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 164, 1952–1960 (2000).
Burns, J.W., Siadat-Pajouh, M., Krishnaney, A.A. & Greenberg, H.B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272, 104–107 (1996).
van Egmond, M., Hanneke van Vuuren, A.J. & van de Winkel, J.G. The human Fc receptor for IgA (Fc alpha RI, CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis. Immunol. Lett. 68, 83–87 (1999).
Balu, S. et al. A novel human IgA monoclonal antibody protects against tuberculosis. J. Immunol. 186, 3113–3119 (2011).
van Spriel, A.B. et al. Effective phagocytosis and killing of Candida albicans via targeting Fc gammaRI (CD64) or Fc alphaRI (CD89) on neutrophils. J. Infect. Dis. 179, 661–669 (1999).
van der Pol, W., Vidarsson, G., Vile, H.A., van de Winkel, J.G. & Rodriguez, M.E. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via Fc alphaRI (CD89). J. Infect. Dis. 182, 1139–1145 (2000).
Kobayashi, T. et al. Effective in vitro clearance of Porphyromonas gingivalis by Fc alpha receptor I (CD89) on gingival crevicular neutrophils. Infect. Immun. 69, 2935–2942 (2001).
Launay, P. et al. Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger′s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J. Exp. Med. 191, 1999–2009 (2000).
Duchez, S. et al. Premature replacement of mu with alpha immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc. Natl Acad. Sci. USA 107, 3064–3069 (2010).
Leibl, H., Tomasits, R. & Mannhalter, J.W. Isolation of human serum IgA using thiophilic adsorption chromatography. Protein Expr. Purif. 6, 408–410 (1995).
Sandin, C. et al. Isolation and detection of human IgA using a streptococcal IgA-binding peptide. J. Immunol. 169, 1357–1364 (2002).
Beyer, T. et al. Serum-free production and purification of chimeric IgA antibodies. J. Immunol. Methods 346, 26–37 (2009).
Kanamaru, Y. et al. Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J. Immunol. 180, 2669–2678 (2008).
Kanamaru, Y. et al. IgA Fc receptor I signals apoptosis through the FcR gamma ITAM and affects tumor growth. Blood 109, 203–211 (2007).
Monteiro, R.C. The role of IgA and IgA Fc receptors as anti-inflammatory agents. J. Clin. Immunol. 30 (Suppl 1), S61–64 (2010).
Blanchard, T.G. et al. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect. Immun. 63, 1394–1399 (1995).
Goto, T. et al. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67, 2531–2539 (1999).
Renegar, K.B. & Small, P.A., Jr . Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 146, 1972–1978 (1991).
Takase, H., Murakami, Y., Endo, A. & Ikeuchi, T. Antibody responses and protection in mice immunized orally against influenza virus. Vaccine 14, 1651–1656 (1996).
Phalipon, A. et al. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182, 769–778 (1995).
Bomsel, M. et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34, 269–280 (2011).
Duval, M., Posner, M.R. & Cavacini, L.A. A bispecific antibody composed of a nonneutralizing antibody to the gp41 immunodominant region and an anti-CD89 antibody directs broad human immunodeficiency virus destruction by neutrophils. J. Virol. 82, 4671–4674 (2008).
Valerius, T. et al. Fc alphaRI (CD89) as a novel trigger molecule for bispecific antibody therapy. Blood 90, 4485–4492 (1997).
Deo, Y.M., Sundarapandiyan, K., Keler, T., Wallace, P.K. & Graziano, R.F. Bispecific molecules directed to the Fc receptor for IgA (Fc alpha RI, CD89) and tumor antigens efficiently promote cell-mediated cytotoxicity of tumor targets in whole blood. J. Immunol. 160, 1677–1686 (1998).
Valerius, T. et al. Activated neutrophils as effector cells for bispecific antibodies. Cancer Immunol. Immunother. 45, 142–145 (1997).
Ma, J.K. et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 4, 601–606 (1998).
Huls, G. et al. Antitumor immune effector mechanisms recruited by phage display-derived fully human IgG1 and IgA1 monoclonal antibodies. Cancer Res. 59, 5778–5784 (1999).
Stockmeyer, B. et al. Triggering Fc alpha-receptor I (CD89) recruits neutrophils as effector cells for CD20-directed antibody therapy. J. Immunol. 165, 5954–5961 (2000).
Dechant, M. & Valerius, T. IgA antibodies for cancer therapy. Crit. Rev. Oncol. Hematol. 39, 69–77 (2001).
Stockmeyer, B. et al. Mechanisms of G-CSF- or GM-CSF-stimulated tumor cell killing by Fc receptor-directed bispecific antibodies. J. Immunol. Methods 248, 103–111 (2001).
Sundarapandiyan, K. et al. Bispecific antibody-mediated destruction of Hodgkin's lymphoma cells. J. Immunol. Methods 248, 113–123 (2001).
van Egmond, M. et al. Enhancement of polymorphonuclear cell-mediated tumor cell killing on simultaneous engagement of Fc gammaRI (CD64) and Fc alphaRI (CD89). Cancer Res. 61, 4055–4060 (2001).
Dechant, M. et al. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood 100, 4574–4580 (2002).
Dechant, M. et al. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J. Immunol. 179, 2936–2943 (2007).
Zhao, J. et al. Recombinant human monoclonal igA antibody against CEA to recruit neutrophils to CEA-expressing cells. Oncol Res. 17, 217–222 (2008).
Guettinger, Y. et al. A recombinant bispecific single-chain fragment variable specific for HLA class II and Fc alpha RI (CD89) recruits polymorphonuclear neutrophils for efficient lysis of malignant B lymphoid cells. J. Immunol. 184, 1210–1217 (2010).
Lohse, S. et al. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J. Immunol. 186, 3770–3778 (2011).
Bakema, J.E. & van Egmond, M. Immunoglobulin A: a next generation of therapeutic antibodies? mAbs. 3, 352–361 (2011).
Bakema, J.E. et al. Targeting the immunoglobulin A Fc receptor (Fc alphaRI) on polymorphonuclear cells induces tumor cell killing through autophagy. J. Immunol. 187, 726–732 (2011).
Martin-Orozco, N. et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009).
Pelletier, M. et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115, 335–343 (2010).
Scapini, P. et al. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177, 195–203 (2000).
Acknowledgements
J.E Bakema and M. van Egmond are supported by the Maag Lever Darm stichting (WO 10-41) and by the Netherlands Organization for Scientific Research (VIDI 016.086.320).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Bakema, J., van Egmond, M. The human immunoglobulin A Fc receptor FcαRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol 4, 612–624 (2011). https://doi.org/10.1038/mi.2011.36
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2011.36
This article is cited by
-
Identification of distinct LRC- and Fc receptor complex-like chromosomal regions in fish supports that teleost leukocyte immune-type receptors are distant relatives of mammalian Fc receptor-like molecules
Immunogenetics (2021)
-
The immune landscape of IgA induction in the gut
Seminars in Immunopathology (2021)
-
Presence of salivary IgA anti-citrullinated protein antibodies associate with higher disease activity in patients with rheumatoid arthritis
Arthritis Research & Therapy (2020)
-
Topical application of human-derived Ig isotypes for the control of acute respiratory infection evaluated in a human CD89-expressing mouse model
Mucosal Immunology (2019)
-
Human IgM antibody rHIgM22 promotes phagocytic clearance of myelin debris by microglia
Scientific Reports (2018)