Abstract
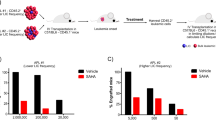
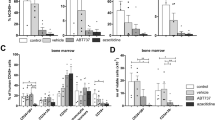
We recently identified that the MEK/ERK1/2 pathway synergized with retinoic acid (RA) to restore both transcriptional activity and RA-induced differentiation in RA-resistant acute promyelocytic leukemia (APL) cells. To target the MEK/ERK pathway, we identified glycogen synthase kinase-3β (GSK-3β) inhibitors including lithium chloride (LiCl) as activators of this pathway in APL cells. Using NB4 (RA-sensitive) and UF-1 (RA-resistant) APL cell lines, we observed that LiCl as well as synthetic GSK-3β inhibitors decreased proliferation, induced apoptosis and restored, in RA-resistant cells, the expression of RA target genes and the RA-induced differentiation. Inhibition of the MEK/ERK1/2 pathway abolished these effects. These results were corroborated in primary APL patient cells and translated in vivo using an APL preclinical mouse model in which LiCl given alone was as efficient as RA in increasing survival of leukemic mice compared with untreated mice. When LiCl was combined with RA, we observed a significant survival advantage compared with mice treated by RA alone. In this work, we demonstrate that LiCl, a well-tolerated agent in humans, has antileukemic activity in APL and that it has the potential to restore RA-induced transcriptional activation and differentiation in RA-resistant APL cells in an MEK/ERK-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Robb L . Cytokine receptors and hematopoietic differentiation. Oncogene 2007; 26: 6715–6723.
Rosenbauer F, Tenen DG . Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol 2007; 7: 105–117.
Collins SJ, Robertson KA, Mueller L . Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-alpha). Mol Cell Biol 1990; 10: 2154–2163.
Grignani F, Fagioli M, Alcalay M, Longo L, Pandolfi PP, Donti E et al. Acute promyelocytic leukemia: from genetics to treatment. Blood 1994; 83: 10–25.
Rochette-Egly C, Germain P . Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl Recept Signal 2009; 7: e005.
Martens JH, Brinkman AB, Simmer F, Francoijs KJ, Nebbioso A, Ferrara F et al. PML-RARalpha/RXR alters the epigenetic landscape in acute promyelocytic leukemia. Cancer Cell 2010; 17: 173–185.
Zhu J, Gianni M, Kopf E, Honoré N, Chelbi-Alix M, Koken M et al. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci USA 1999; 96: 14807–14812.
Lallemand-Breitenbach V, Zhu J, Chen Z, de Thé H . Curing APL through PML/RARA degradation by As2O3. Trends Mol Med 2012; 18: 36–42.
Adès L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood 2010; 115: 1690–1696.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369: 111–121.
Imaizumi M, Suzuki H, Yoshinari M, Sato A, Saito T, Sugawara A et al. Mutations in the E-domain of RAR portion of the PML/RAR chimeric gene may confer clinical resistance to all-trans retinoic acid in acute promyelocytic leukemia. Blood 1998; 92: 374–382.
Schachter-Tokarz E, Kelaidi C, Cassinat B, Chomienne C, Gardin C, Raffoux E et al. PML-RARalpha ligand-binding domain deletion mutations associated with reduced disease control and outcome after first relapse of APL. Leukemia 2010; 24: 473–476.
Cassinat B, Zassadowski F, Ferry C, Llopis L, Bruck N, Lainey E et al. New role for granulocyte colony-stimulating factor-induced extracellular signal-regulated kinase 1/2 in histone modification and retinoic acid receptor α recruitment to gene promoters: relevance to acute promyelocytic leukemia cell differentiation. Mol Cell Biol 2011; 31: 1409–1418.
Lalevée S, Ferry C, Rochette-Egly C . Phosphorylation control of nuclear receptors. Methods Mol Biol 2010; 647: 251–266.
Zassadowski F, Rochette-Egly C, Chomienne C, Cassinat B . Regulation of the transcriptional activity of nuclear receptors by the MEK/ERK1/2 pathway. Cell Signal 2012; 24: 2369–2377.
Doble BW, Woodgett JR . GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116: 1175–1186.
McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Abrams SL, Montalto G et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia 2014; 28: 15–33.
Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M . Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med 2006; 12: 89–98.
Huang J, Zhang Y, Bersenev A, O'Brien WT, Tong W, Emerson SG et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin Invest 2009; 119: 3519–3529.
Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML . Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature 2008; 455: 1205–1209.
Si J, Mueller L, Collins SJ . GSK3 inhibitors enhance retinoic acid receptor activity and induce the differentiation of retinoic acid-sensitive myeloid leukemia cells. Leukemia 2011; 25: 1914–1918.
Gupta K, Gulen F, Sun L, Aguilera R, Chakrabarti A, Kiselar J et al. GSK3 is a regulator of RAR-mediated differentiation. Leukemia 2012; 26: 1277–1285.
Cassinat B, de Botton S, Kelaidi C, Ades L, Zassadowski F, Guillemot I et al. When can real-time quantitative RT-PCR effectively define molecular relapse in acute promyelocytic leukemia patients? (Results of the French Belgian Swiss APL Group). Leukemia Res 2009; 33: 1178–1182.
Cassinat B, Chevret S, Zassadowski F, Balitrand N, Guillemot I, Menot ML et al. In vitro all-trans retinoic acid sensitivity of acute promyelocytic leukemia blasts: a novel indicator of poor patient outcome. Blood 2001; 98: 2862–2864.
Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG et al. A PMLRARalpha transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci USA 1997; 94: 2551–2556.
Padua RA, Larghero J, Robin M, le Pogam C, Schlageter MH, Muszlak S et al. PML-RARA-targeted DNA vaccine induces protective immunity in a mouse model of leukemia. Nat Med 2003; 9: 1413–1417.
Pasquali L, Busceti CL, Fulceri F, Paparelli A, Fornai F . Intracellular pathways underlying the effects of lithium. Behav Pharmacol 2010; 21: 473–492.
Calabresse C, Barbey S, Venturini L, Balitrand N, Degos L, Fenaux P et al. In vitro treatment with retinoids or the topoisomerase inhibitor, VP-16, evidences different functional apoptotic pathways in acute promyelocytic leukemic cells. Leukemia 1995; 9: 2049–2057.
Lallemand-Breitenbach V, Guillemin MC, Janin A, Daniel MT, Degos L, Kogan SC et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med 1999; 189: 1043–1052.
Leroy P, Nakshatri H, Chambon P . Mouse retinoic acid receptor alpha 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc Natl Acad Sci USA 1991; 88: 10138–10142.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–789.
Yan XB, Hou HL, Wu LM, Liu J, Zhou JN . Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology 2007; 53: 487–495.
Kopnisky KL, Chalecka-Franaszek E, Gonzalez-Zulueta M, Chuang DM . Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activities. Neuroscience 2003; 116: 425–435.
Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS et al. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem 2004; 89: 324–336.
Rehani K, Wang H, Garcia CA, Kinane DF, Martin M . Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. J Immunol 2009; 182: 547–553.
Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS . Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med 2012; 18: 1778–1785.
Luo J . Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett 2009; 273: 194–200.
Rössig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S . Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem 2002; 277: 9684–9689.
McQueen J, van Dyk D, Young B, Loewen C, Measday V . The Mck1 GSK-3 kinase inhibits the activity of Clb2-Cdk1 post-nuclear division. Cell Cycle 2012; 11: 3421–3432.
Abrahamsson AE, Geron I, Gotlib J, Dao KH, Barroga CF, Newton IG et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA 2009; 106: 3925–3929.
Banerji V, Frumm SM, Ross KN, Li LS, Schinzel AC, Hahn CK et al. The intersection of genetic and chemical genomic screens identifies GSK-3α as a target in human acute myeloid leukemia. J Clin Invest 2012; 122: 935–947.
Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH et al. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell 2010; 17: 597–608.
Rice AM, Holtz KM, Karp J, Rollins S, Sartorelli AC . Analysis of the relationship between Scl transcription factor complex protein expression patterns and the effects of LiCl on ATRA-induced differentiation in blast cells from patients with acute myeloid leukemia. Leukemia Res 2004; 28: 1227–1237.
Song EY, Palladinetti P, Klamer G, Ko KH, Lindeman R, O'Brien TA et al. Glycogen synthase kinase-3β inhibitors suppress leukemia cell growth. Exp Hematol 2010; 38: 908–921.
Wang R, Xia L, Gabrilove J, Waxman S, Jing Y . Downregulation of Mcl-1 through GSK-3β activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia 2013; 27: 315–324.
Acknowledgements
This study was supported by a grant from Ligue Contre le Cancer (RS10/75-61) and an ANR Grant BiotecS 2009 ‘GSKforonco’. We thank Targeon SAS for the kind gift of GSK-3β inhibitors. We are grateful to Gil Letort for precious technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Zassadowski, F., Pokorna, K., Ferre, N. et al. Lithium chloride antileukemic activity in acute promyelocytic leukemia is GSK-3 and MEK/ERK dependent. Leukemia 29, 2277–2284 (2015). https://doi.org/10.1038/leu.2015.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.159
This article is cited by
-
GSK-3: a multifaceted player in acute leukemias
Leukemia (2021)
-
Lithium Reversibly Inhibits Schwann Cell Proliferation and Differentiation Without Inducing Myelin Loss
Molecular Neurobiology (2017)
-
Lithium Chloride Facilitates Autophagy Following Spinal Cord Injury via ERK-dependent Pathway
Neurotoxicity Research (2017)
-
Effect of ATRA and ATO on the expression of tissue factor in NB4 acute promyelocytic leukemia cells and regulatory function of the inflammatory cytokines TNF and IL-1β
Annals of Hematology (2017)
-
pVAX14DNA-mediated add-on immunotherapy combined with arsenic trioxide and all-trans retinoic acid targeted therapy effectively increases the survival of acute promyelocytic leukemia mice
Blood Cancer Journal (2015)