Abstract
Phospholipase C (PLC) ɛ is a phosphoinositide-specific PLC regulated by small GTPases including Ras and Rap. We previously demonstrated that PLCɛ has an important role in the development of phorbol ester-induced skin inflammation. In this study, we investigated the role of PLCɛ in ultraviolet (UV) B-induced acute inflammatory reactions in the skin. Wild-type (PLCɛ+/+) and PLCɛ gene knockout (PLCɛ−/−) mice were irradiated with a single dose of UVB at 1, 2.5, and 10 kJ/m2 on the dorsal area of the skin, and inflammatory reactions in the skin were histologically evaluated up to 168 h after irradiation. In PLCɛ+/+ mice, irradiation with 1 and 2.5 kJ/m2 UVB resulted in dose-dependent neutrophil infiltration in the epidermis at 24 and 48 h after irradiation. When mice were irradiated with 10 kJ/m2 of UVB, most mice developed skin ulcers by 48 h and these ulcers became more severe at 168 h. In PLCɛ−/− mice, UVB (1 or 2.5 kJ/m2)-induced neutrophil infiltration was markedly suppressed compared with PLCɛ+/+ mice. The suppression of neutrophil infiltration in PLCɛ−/− mice was accompanied by attenuation of UVB-induced production of CXCL1/keratinocyte-derived chemokine (KC), a potent chemokine for neutrophils, in the whole skin. Cultured epidermal keratinocytes and dermal fibroblasts produced CXCL1/KC in a PLCɛ-dependent manner after UVB irradiation, and the UVB-induced upregulation of CXCL1/KC in these cells was significantly abolished by a PLC inhibitor. Furthermore, UVB-induced epidermal thickening was noticeably reduced in the skin of PLCɛ−/− mice. These results indicate that PLCɛ has a crucial role in UVB-induced acute inflammatory reactions such as neutrophil infiltration and epidermal thickening by at least in part regulating the expression of CXCL1/KC in skin cells such as keratinocytes and fibroblasts.
Similar content being viewed by others
Main
Solar ultraviolet (UV) radiation induces various acute effects on the skin and most of the harmful biological effects of UV are attributed to UVB (290–320 nm).1, 2, 3, 4, 5 The acute effects of UVB include inflammation, apoptosis, gene mutation, and immunosuppression.1, 2, 3, 4 The inflammation caused by UVB has been well-documented clinically and histologically. Clinically, UVB-induced skin inflammation results in cutaneous injury such as erythema, heat, swelling, and pain.1, 2, 6 Histologically, skin inflammation caused by UVB is characterized by dilation of blood vessels,1, 2 infiltration of neutrophils,1, 7, 8, 9, 10 and epidermal thickening.2, 9, 11, 12, 13 Biochemical changes including cytokine expression are also induced during UVB-induced acute skin inflammation.1 UVB-induced skin inflammatory reactions constitute a potent tumor-promoting event that contributes to photocarcinogenesis.14, 15, 16 Thus, development of a method for regulating UVB-induced acute effects is important for protection against photocarcinogenesis. However, the molecular mechanisms by which UVB induces acute effects on the skin have not been fully clarified.
Phospholipase C (PLC) ɛ is one of the members of the phosphoinositide-specific PLC family that catalyzes the hydrolysis of a membrane phospholipid, phosphatidylinositol 4,5-bisphosphate, to generate two important second messengers, diacylglycerol and inositol 1,4,5-trisphosphate.17 Diacylglycerol and inositol 1,4,5-trisphosphate induce the activation of protein kinase C (PKC) and mobilization of calcium from intracellular stores, respectively.18, 19 PLCɛ was identified by us19, 20 and other groups21, 22 as a downstream effector of the Ras family small GTPases. It has been suggested that PLCɛ mediates diverse signals from outside the cell because of the multiple regulatory mechanisms of the enzyme.17 Pathophysiological roles of PLCɛ have been studied in various animal models carrying artificial or spontaneous mutations in the chromosomal gene.23, 24, 25 Also, using positional cloning, the human PLCɛ gene PLCE1 was identified as a causal gene for nephrotic syndrome.26 However, few studies on the role of PLCɛ in external stimuli-induced pathophysiological conditions have been carried out.
We previously demonstrated that PLCɛ has an important role in the development of phorbol ester-induced skin inflammation.27 The purpose of this study was to investigate the involvement of PLCɛ in UVB-induced acute inflammation in the skin using PLCɛ−/− mice. We show that PLCɛ has a crucial role in UVB-induced neutrophil infiltration in the skin by regulating expression of CXCL1/keratinocyte-derived chemokine (KC), a potent chemokine for neutrophils. We also demonstrate that PLCɛ regulates UVB-induced epidermal thickening.
MATERIALS AND METHODS
Chemicals
A PLC inhibitor U73122 and a tumor necrosis factor (TNF)-α converting enzyme (TACE) inhibitor TAPI-2 were purchased from Calbiochem (La Jolla, CA, USA) and BIOMOL International (Plymouth Meeting, PA, USA), respectively. Dimethyl dulfoxide (DMSO) was obtained from Nacalai Tesque (Kyoto, Japan).
Mice
PLCɛ−/− mice (129/Sv × C57BL/6 mixed background) were developed as described previously.28 PLCɛ+/+ and PLCɛ−/− mice were obtained by mating with PLCɛ+/− male and PLCɛ+/− female animals. The care and use of the mice was reviewed and approved by the Institutional Animal Committee of Kobe University Graduate School of Medicine. Mice were housed under special pathogen-free conditions and all animal experiments were conducted according to the ‘Guideline for Animal Experimentation at Kobe University Graduate School of Medicine’.
UVB Irradiation and Assessment of its effects on Skin
A bank of six TL 20W/12RS fluorescent lamps (Philips, Eindhoven, Holland) was used to irradiate mice.29 These lamps emit a continuous spectrum from 275 to 390 nm, with a peak emission at 313 nm; ∼65% of that radiation is within the UVB wave range. The irradiance was 3.8 J/m2 per second at a distance of 40 cm as measured by a UVR-305/365D digital radiometer (Tokyo Kogaku Kikai KK, Tokyo, Japan). For the study of UVB-induced skin inflammation, 12–15-week-old mice were selected and divided into two groups (PLCɛ+/+ or PLCɛ−/−) of 10 mice (5 male mice; 5 female mice). The day before UVB irradiation, dorsal areas of the skin were shaved. Mice were irradiated with a single dose of 1, 2.5, or 10 kJ/m2 UVB, and biopsied at the indicated time points.
Histology and Immunostaining
Biopsied skins were fixed with 10% neutralized formalin, embedded in paraffin, and then stained with hematoxylin and eosin (H&E) or anti-proliferating cell nuclear antigen (PCNA) antibody (M0879; Dako Cytomation, Copenhagen, Denmark). For statistical studies, the total number of neutrophils in neutrophil microabscesses in the epidermis in each section was counted and the density of neutrophils in the epidermis was expressed as the mean number of neutrophils/100 μm length of epidermis. The thickness of the epidermis (distance from the top of the basement membrane to the bottom of the stratum corneum) and percentage of PCNA-positive cells among total basal cells in the basal cell layer were measured in five fields per sample and averaged.
Identification of Neutrophils
Neutrophils were identified on H&E-stained skin sections. Specifically, pink-stained leukocytes with nuclei definitely divided into two to three lobes were identified as neutrophils. The identification of neutrophils was performed also with immunostaining using anti-Gr-1 antibody (MAB; 1037, R&D Systems, Minneapolis, MN, USA) on frozen sections (Supplementary Figure 1).
Quantitative Real-Time PCR (qRT–PCR)
Total cellular RNA isolation using Trizol (Invitrogen, Carlsbad, CA, USA) and cDNA synthesis were performed as described previously.27 qRT–PCR was carried out using the SYBR Premix Ex Taq II kit (Takara Bio, Kyoto, Japan) with the Thermal Cycler Dice Real-Time System (Takara Bio). Relative mRNA levels were determined using the comparative Ct method followed by normalizing to the β-actin mRNA level in each cDNA sample. The sequence of the primers were 5′-GCTTGTTCAGTTTAAAGATGGTAGGC-3′ and 5′-CGTGTTGACCATACAATATGAAAGACG-3′ for CXCL1/KC, 5′-AGCCCACGTCGTAGCAAACCACCAA-3′ and 5′-ACACCCATTCCCTTCACAGAGCAAT-3′ for TNF-α, and 5′-CTACAATGAGCTGCCTGTGG-3′ and 5′-CAACGTCACACTTCATGATGG-3′ for β-actin.
Primary Cultures of Epidermal Keratinocytes and Dermal Fibroblasts
Primary cultures of epidermal keratinocytes and dermal fibroblasts were performed as described previously30 with minor modifications. Briefly, epidermal keratinocytes were isolated from the dorsal skin of newborn mice at postnatal day 1 and seeded with defined keratinocyte-SFM supplemented with growth factors onto plastic plates coated with type 1-A collagen (Nitta gelatin, Osaka, Japan). Dermal fibroblasts were isolated from the dorsal skin of newborn mice at postnatal day 1 and cultured on ordinary culture plates in DMEM supplemented with 10% FBS.
CXCL1/KC Determination by ELISA
CXCL1/KC protein content in the culture medium was measured using a Mouse CXCL1/KC ELISA Kit (Biosource, Camarillo, CA, USA) according to the manufacturer's instruction.
Statistical Analysis
Statistical differences in the data between PLCɛ+/+ and PLCɛ−/− mice were determined using Student's t-test. P<0.05 was considered to be statistically significant.
RESULTS
UVB Induces Neutrophil infiltration in the Skin
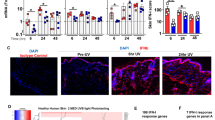
As an initial step of investigating whether PLCɛ has a role in UVB-induced acute skin inflammation, we irradiated the skin of PLCɛ+/+ and PLCɛ−/− mice with a single dose of UVB at 1 kJ/m2, and examined neutrophil infiltration in the skin as a representative UVB-induced acute inflammatory reaction at 0, 3, 6, 12, 18, 24, 48, and 168 h after irradiation (Figure 1). Before UVB irradiation, no neutrophil infiltration was detected in the skin of either PLCɛ+/+ or PLCɛ−/− mice (Figures 1a and g). When mice were irradiated with 1 kJ/m2 of UVB, neutrophils were first observed at 12 h mainly in the blood vessels located in the subcutaneous fat tissue of PLCɛ+/+ mice (Figure 1b). Neutrophil infiltration became more severe at 18 h and neutrophils were present not only in blood vessels but also in the dermal stroma (Figure 1c). No apparent neutrophil infiltration was found in the skins of PLCɛ+/+ mice collected at 3 or 6 h after irradiation (data not shown). In skin sections of PLCɛ+/+ mice collected at 24 and 48 h, infiltration of neutrophils was present in the epidermis, most of which resulted in the formation of patchy neutrophil microabscesses (Figures 1d and e; Supplementary Figure 1). Neutrophil infiltration in the epidermis disappeared almost completely by 168 h in PLCɛ+/+ mice (Figure 1f). Although neutrophil infiltration was observed in the dermis at 24 and 48 h in PLCɛ+/+ mice, the extent of the infiltration was much less than that in the epidermis. In contrast, in PLCɛ−/− mice, no neutrophil microabscesses were found in the epidermis at any time point examined (Figures 1h and i).
Histological findings of the time course of neutrophil infiltration in the epidermis of PLCɛ+/+ and PLCɛ−/− mice irradiated with 1 kJ/m2 of UVB. The shaved dorsal skins of PLCɛ+/+ and PLCɛ−/− mice were irradiated with a single dose of UVB at 1 kJ/m2 (n=10 for each PLCɛ background, 5 male mice and 5 female mice). Skin specimens were prepared at 0, 12, 18, 24, 48, and 168 h after irradiation, and were stained with H&E. Representative photographs of skin sections from PLCɛ+/+ (+/+) and PLCɛ−/− (−/−) mice are shown. Insets in (b) and (c) show a blood vessel containing neutrophils and neutrophil infiltration in the stroma of the dermis, respectively. Neutrophil microabscesses are surrounded by dotted lines in (d) and (e). Bar, 100 μm.
UVB-Induced Neutrophil Infiltration in the Skin is Suppressed in PLCɛ−/− Mice
We further assessed the effect of higher UVB doses, 2.5 and 10 kJ/m2, on neutrophil infiltration in the skins of PLCɛ+/+ and PLCɛ−/− mice at 24 and 48 h. When mice were irradiated with 2.5 kJ/m2 of UVB, the PLCɛ+/+ mice and some PLCɛ−/− mice showed epidermal neutrophil microabscess(es) at 24 and/or 48 h. When mice were irradiated with 10 kJ/m2 of UVB, most mice developed skin ulcers by 48 h irrespective of the PLCɛ background and these ulcers became more severe at 168 h (data not shown). After irradiation with 1 and 2.5 kJ/m2 of UVB, the density of infiltrated neutrophils in PLCɛ−/− mice was significantly lower than in PLCɛ+/+ mice at 24 and 48 h (Figure 2). Based on these results, 1 kJ/m2 was considered to be a suitable UVB dose for investigating the difference in UVB-induced skin inflammation between PLCɛ+/+ and PLCɛ−/− mice, and was used in all further studies.
Comparison of the density of neutrophils in the epidermis of PLCɛ+/+ and PLCɛ−/− mice irradiated with UVB. The shaved dorsal skins of PLCɛ+/+ (white bar) and PLCɛ−/− (black bar) mice were irradiated with a single dose of UVB at 2.5 kJ/m2 (n=10 for each PLCɛ background, 5 male mice and 5 female mice). Skin specimens were prepared at 24 and 48 h after irradiation, and were stained with H&E. The density of neutrophils in the epidermis at each time point was calculated together with samples used in Figure 1 as described in Materials and methods. Data are expressed as mean±s.d. *P<0.05; **P<0.01.
UVB-Induced Production of CXCL1/KC is Attenuated in the Skin of PLCɛ−/− Mice
We next investigated the cause of attenuated neutrophil infiltration in UVB-treated PLCɛ−/− mice skin. The possibility that attenuated neutrophil infiltration in PLCɛ−/− mice is due to a reduction in PLCɛ-dependent chemotaxis in neutrophils was ruled out because the cells do not express PLCɛ.27 Infiltration of neutrophils in the inflammatory site is controlled by chemokines.31, 32 It has been shown that, among the various chemokines, CXCL8/interleukin (IL)-8 and CXCL1/KC, a functional mouse homolog of human CXCL8/IL-8, have a pivotal role in the mediation of UVB-induced inflammation in the human skin.10, 33, 34, 35 Thus, we examined the UVB-induced changes in CXCL1/KC expression in the skin of PLCɛ+/+ and PLCɛ−/− mice by qRT–PCR (Figure 3a). Similar results were obtained at UVB doses of 1.0 and 2.5 kJ/m2. In PLCɛ+/+ mice, UVB irradiation resulted in an increase in CXCL1/KC mRNA expression up to 24 h. In contrast, in the skin of PLCɛ−/− mice, CXCL1/KC mRNA levels at each time point were much lower than those in PLCɛ+/+ mice, although expression of CXCL1/KC mRNA was also enhanced by UVB irradiation in the skin. To identify skin cell type(s) that produce CXCL1/KC in response to UVB cultured keratinocytes and fibroblasts were irradiated with UVB and protein levels of CXCL1/KC in culture supernatants were analyzed by ELISA (Figure 3b). The levels of CXCL1/KC in the supernatants of non-irradiated keratinocytes and fibroblasts from PLCɛ+/+ mice were significantly higher than those from PLCɛ−/− mice. UVB irradiation resulted in an increase in this chemokine in the supernatants of both cell types irrespective of PLCɛ genotypes and the levels of CXCL1/KC in the supernatants of both of the cell types from PLCɛ+/+ mice were much higher than those from PLCɛ−/− mice. Evidence for the involvement of PLCɛ in the UVB-induced production of CXCL1/KC in keratinocytes and fibroblasts was further supported by the observation that a broad spectrum PLC inhibitor U73122 significantly suppressed the UVB-induced upregulation of CXCL1/KC in these cells from PLCɛ+/+ mice (Figure 3c). It has been demonstrated that UVB is a potent stimulator of TNF-α production in the skin.36 Also, TNF-α is known to induce CXCL1/KC production in some cell types.37 These reports raise the possibility that UVB upregulates CXCL1/KC production through enhancing TNF-α production in skin cells. Thus, we examined the possible involvement of TNF-α in UVB-induced CXCL1/KC production. No alteration in the level of TNF-α mRNA in the skin after UVB irradiation between PLCɛ+/+ and PLCɛ−/− mice was observed (Figure 4a). In addition, a TACE inhibitor, TAPI-2, did not affect the levels of CXCL1/KC in the supernatants of either keratinocytes or fibroblasts from PLCɛ+/+ mice after UVB irradiation (Figure 4b). Thus, an indirect effect of UVB through TNF-α on CXCL1/KC production was ruled out.
UVB-induced expression of CXCL1/KC in the skin of PLCɛ+/+ and PLCɛ−/− mice. (a) The shaved dorsal skins of PLCɛ+/+ (white bar) and PLCɛ−/− (black bar) mice were irradiated with a single dose of UVB at either 1 or 2.5 kJ/m2. The CXCL/1KC mRNA level in the skin collected at 0, 3, 6, 12, and 24 h was quantitated by qRT–PCR. The results are expressed as the fold change using the value of the non-irradiated skin as 1. **P <0.01. (b) Cultured keratinocytes and fibroblasts from were irradiated with a single dose of UVB at 0.1 kJ/m2 followed by culturing for another 24 h. Medium was removed before the UVB treatment. CXCL1/KC secretion in the supernatants was quantitated by ELISA and was calculated as pg/ml/105 cells/24 h. (c) After pre-treatment with 2.5 μM U73122 or 0.1% DMSO for 10 min, cultured keratinocytes and fibroblasts from PLCɛ+/+ mice were irradiated with a single dose of UVB at 1 kJ/m2 followed by culturing for another 24 h in the presence of either 2.5 μM U73122 or 0.1% DMSO. CXCL1/KC secretion in the supernatants was quantitated by ELISA and was calculated as pg/ml/105 cells/24 h. The results shown are representative of three independent experiments.
Role of TNF-α in UVB-induced expression of CXCL1/KC in skin cells. (a) The shaved dorsal skins of PLCɛ+/+ (white bar) and PLCɛ−/− (black bar) mice were irradiated with a single dose of UVB at 1 kJ/m2. The TNF-α mRNA level in the skin collected at 0, 3, 6, 12, and 24 h was quantitated by qRT–PCR. The results are expressed as the fold change using the value of the non-irradiated skin as 1. (b) After pretreatment without or with 200 nM TAPI-2 for 1 h, cultured keratinocytes and fibroblasts from PLCɛ+/+ mice were irradiated with a single dose of UVB at 1 kJ/m2 followed by culturing for another 24 h in the absence or presence of 200 nM TAPI-2. CXCL1/KC secretion in the supernatants was quantitated by ELISA and was calculated as pg/ml/105 cells/24 h. The results shown are representative of three independent experiments.
UVB-Induced Epidermal Thickening is Reduced in the Skin of PLCɛ−/− Mice
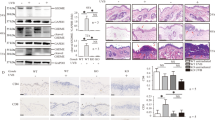
We next investigated the effect of PLCɛ deficiency on the UVB-induced proliferative response of the epidermis. At 168 h after irradiation with 1 kJ/m2 of UVB, PLCɛ+/+ mouse skin showed a marked increase in epidermal thickness (Figure 1f, Figures 5a and b). UVB-induced epidermal thickening was significantly suppressed in PLCɛ−/− mice. In non-irradiated epidermis, proliferating cells positive for PCNA constituted 3–4% of basal cells in the basal cell layer of the epidermis, and there was no apparent difference in the number of PCNA-positive cells between PLCɛ+/+ and PLCɛ−/− mice.28 UVB irradiation noticeably increased the number of PCNA-positive cells in the basal cells in PLCɛ+/+ mice, and the percentage of PCNA-positive cells among the basal cells reached ∼60% at 168 h after irradiation (Figure 5b). In contrast, the UVB-induced increase in the number of PCNA-positive cells was markedly lower at ∼10% in PLCɛ−/− mice.
Suppression of the UVB-induced proliferative response in PLCɛ−/− mice. (a) The epidermal thickness of PLCɛ+/+ (white bar) and PLCɛ−/− (black bar) mice at 0 and 168 h after irradiation with a single dose of UVB at 1 kJ/m2 used in Figure 1 was calculated as described in materials and methods. Data are expressed as mean±s.d. *P<0.05. (b) The skin sections used in (a) were stained with the anti-PCNA antibody. Representative photographs of skin sections from PLCɛ+/+ (+/+) and PLCɛ−/− (−/−) mice at 168 h after irradiation are shown (upper two panels). Bar, 100 μm. The percentage of PCNA-positive cells in the basal cell layer against total basal cells in skin sections from PLCɛ+/+ (+/+) and PLCɛ−/− (−/−) mouse was determined as described in materials and methods and is expressed as mean±s.d. (lower panel). **P<0.01.
DISCUSSION
In the present study, we have shown that the extent of UVB-induced neutrophil infiltration in the mouse skin is closely associated with the level of expression of CXCL1/KC in the skin. Furthermore, our study identified a novel function of PLCɛ as a critical molecule regulating UVB-induced CXCL1/KC production. Thus, it is considered that UVB-induced neutrophil infiltration is regulated at least in part by PLCɛ-mediated CXCL1/KC expression. Furthermore, the UVB-induced proliferative response in the epidermis is suppressed in PLCɛ−/− mice. Collectively, our results demonstrate that UVB-activated PLCɛ has a crucial role in UVB-induced acute inflammatory reactions.
The molecular mechanism of UVB-induced PLCɛ activation and the downstream signaling pathways from PLCɛ to neutrophil infiltration in the epidermis and epidermal thickening remain to be elucidated. PLCɛ is activated by a variety of mechanisms via multiple upstream regulators including GTPases such as Ras, Rap1, Rap2,17, 20, 21, 22 and RhoA.17, 20, 21, 22 Thus, further investigation of the interaction between UVB and these regulators is necessary to reveal the exact mechanism of UVB-induced PLCɛ activation. As activated PLCɛ produces the PKC activator, diacylglycerol, it is possible that PLCɛ stimulates UVB-induced epidermal neutrophil infiltration and epidermal thickening through a PKC-mediated mechanism. It has been shown that activation of PKCα promotes neutrophil infiltration in the epidermis.38, 39 Also, activation of PKCɛ40 and PKCμ41 has been demonstrated to increase keratinocyte growth.
UVB irradiation at 1.0 and 2.5 kJ/m2 induced upregulation of CXCL1/KC in the skins of the mice. It has been demonstrated that the depth of UVB (1.25–4 kJ/m2) penetration in the mouse dorsal skin is the upper dermis.42 Thus, the UVB-induced increase in CXCL1/KC mRNA in Figure 3a, at least in the case of UVB at 2.5 kJ/m2, was considered to reflect the result of the mRNA from UVB-irradiated epidermis and dermis. In addition, cultured keratinocytes and dermal fibroblasts responded to UVB to produce CXCL1/KC in our study. These results suggest that UVB directly acts on at least keratinocytes and fibroblasts in the mouse skin to produce CXCL1/KC.
Neutrophil infiltration is observed in a number of other human skin diseases besides UVB-induced skin, including psoriasis43 and neutrophilc dermatoses.44 It is well recognized that IL-8-mediated neutrophil infiltration modifies the inflammation process in psoriasis,43 and it has been shown that CXCL1/KC is involved in psoriasis-like skin inflammation in an animal model.45 We previously showed that IL-8 is overexpressed in the skin of patients with pyoderma gangrenosum, a representative of neutrophilc dermatoses.46 We also showed that overexpression of IL-8 using adenovirus vector in human skin grafted on severe combined immunodeficiency mice resulted in neutrophil-associated skin ulcer resembling pyoderma gangrenosum.46 These findings indicate that IL-8-induced neutrophil infiltration has a role either directly or indirectly in the pathogenesis of several inflammatory skin diseases. As IL-8 is not produced in normal conditions, an increased amount of IL-8 in these diseases must result from dysregulation of IL-8 production. Our findings identify a novel function for PLCɛ as a critical molecule regulating UVB-induced neutrophil infiltration in the skin by at least in part regulating the expression of CXCL1/KC in skin cells such as keratinocytes and fibroblasts. It is important to investigate whether PLCɛ activation and its link to the production of CXCL8/IL-8 also has a role in these human skin diseases.
References
Soter NA . Acute effects of ultraviolet radiation on the skin. Semin Dermatol 1990;9:11–15.
Hruza LL, Pentland AP . Mechanisms of UV-induced inflammation. J Invest Dermatol 1993;100:35S–41S.
Ichihashi M, Ueda M, Budiyanto A, et al. UV-induced skin damage. Toxicology 2003;189:21–39.
Nishigori C . Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci 2006;5:208–214.
Beissert S, Loser K . Molecular and cellular mechanisms of photocarcinogenesis. Photochem Photobiol 2008;84:29–34.
Young AR . Acute effect of UVR on human eyes and skin. Biophys Mol Biol 2006;92:80–85.
Hawk JLM, Murphy GM, Holden CA . The presence of neutrophils in human cutaneous ultraviolet-B inflammation. Br J Dermatol 1988;118:27–30.
Cooper KD, Duraiswamy N, Hammerberg C, et al. Neutrophils, differentiated macrophages, and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury. J Invest Dermatol 1993;101:155–163.
Lu YP, Lou YR, Yen P, et al. Time course for early adaptive responses to ultraviolet B light in the epidermis of SKH-1 mice. Cancer Res 1999;59:4591–4602.
Strickland I, Rhodes LE, Flanagan BF, et al. TNF-α and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol 1997;108:763–768.
Seike M, Ikeda M, Morimoto A, et al. Increased synthesis of calcitonin gene-related peptide stimulates keratinocyte proliferation in murine UVB-irradiated skin. J Dermatol Sci 2002;28:135–143.
González S, Astner S, An W, et al. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J Invest Dermatol 2003;121:399–405.
Sano S, Chan KS, Kira M, et al. Signal transducer and activator of transcription 3 is a key regulator of keratinocyte survival and proliferation following UV irradiation. Cancer Res 2005;65:5720–5729.
Wilgus TA, Koki AT, Zweifel BS, et al. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog 2003;38:49–58.
Meeran SM, Punathil T, Katiyar SK . IL-12 deficiency exacerbates inflammatory responses in UV-irradiated skin and skin tumors. J Invest Dermatol 2008;128:2716–2727.
Zheng D, Bode AM, Zhao Q, et al. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res 2008;68:3992–3998.
Bunney TD, Katan M . Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol 2006;16:640–648.
Rhee SG . Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 2001;70:281–312.
Shibatohge M, Kariya K, Liao Y, et al. Identification of PLC210, a Caenorhabditis elegans phospholipase C, as a putative effector of Ras. J Biol Chem 1998;273:6218–6222.
Song C, Hu CD, Masago M, et al. Regulation of a novel human phospholipase C, PLCɛ, through membrane targeting by Ras. J Biol Chem 2001;276:2752–2757.
Kelley GG, Reks SE, Ondrako JM, et al. Phospholipase Cɛ: a novel Ras effector. EMBO J 2001;20:743–754.
Lopez I, Mak EC, Ding J, et al. A novel bifunctional phospholipase C that is regulated by Gα12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem 2001;276:2758–2765.
Kariya K, Bui YK, Gao X, et al. Phospholipase Cɛ regulates ovulation in Caenorhabditis elegans. Dev Biol 2004;274:201–210.
Tadano M, Edamatsu H, Minamisawa S, et al. Congenital semilunar valvulogenesis defect in mice deficient in phospholipase Cɛ. Mol Cell Biol 2005;25:2191–2199.
Wang H, Oestreich EA, Maekawa N, et al. Phospholipase C ɛ modulates β-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circ Res 2005;97:1305–1313.
Hinkes B, Wiggins RC, Gbadegesin R, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet 2006;38:1397–1405.
Ikuta S, Edamatsu H, Li M, et al. Crucial role of phospholipase Cɛ in skin inflammation induced by tumor-promoting phorbol ester. Cancer Res 2008;68:64–72.
Bai Y, Edamatsu H, Maeda S, et al. Crucial role of phospholipase Cɛ in chemical carcinogen-induced skin tumor development. Cancer Res 2004;64:8808–8810.
Kunisada M, Sakumi K, Tominaga Y, et al. 8-Oxoguanine formation induced by chronic UVB exposure makes Ogg1 knockout mice susceptible to skin carcinogenesis. Cancer Res 2005;65:6006–6010.
Hu L, Edamatsu H, Takenaka N, et al. Crucial role of phospholipase Cɛ in induction of local skin inflammatory reactions in the elicitation stage of allergic contact hypersensitivity. J Immunol 2010;184:993–1002.
Oppenheim JJ, Zachariae CO, Mukaida N, et al. Properties of the novel proinflammatory supergene ‘intercrine’ cytokine family. Annu Rev Immunol 1991;9:617–648.
Baggiolini M, Dewald B, Moser B . Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol 1994;55:97–197.
Kondo S, Kono T, Sauder DN, et al. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol 1993;101:690–694.
Pernet I, Sagot V, Schmitt D, et al. UVA1 and UVB radiation but not PGE2 stimulate IL-8 release in normal human keratinocytes. Arch Dermatol Res 1999;291:527–529 .
Storey A, McArdle F, Friedmann PS, et al. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-α-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol 2005;124:248–255.
Bashir MM, Sharma MR, Werth VP . TNF-α production in the skin. Arch Dermatol Res 2009;301:87–91.
Son DS, Parl AK, Rice VM, et al. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther 2007;6:e1–e11.
Cataisson C, Joseloff E, Murillas R, et al. Activation of cutaneous protein kinase Cα induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol 2003;171:2703–2713.
Wang HQ, Smart RC . Overexpression of protein kinase C-α in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-α expression but not tumor promotion. J Cell Sci 1999;112:3497–3506.
Papp H, Czifra G, Bodó E, et al. Opposite roles of protein kinase C isoforms in proliferation, differentiation, apoptosis, and tumorigenicity of human HaCaT keratinocytes. Cell Mol Life Sci 2004;61:1095–1105.
Rennecke J, Rehberger PA, Fürstenberger G, et al. Protein-kinase-Cμ expression correlates with enhanced keratinocyte proliferation in normal and neoplastic mouse epidermis and in cell culture. Int J Cancer 1999;80:98–103.
Takeuchi S, Zhang W, Wakamatsu K, et al. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc Natl Acad Sci 2004;101:15076–15081.
Lowes MA, Bowcock AM, Krueger JG . Pathogenesis and therapy of psoriasis. Nature 2007;445:866–873.
Saavedra AP, Kovacs SC, Moschella SL . Neutrophilic dermatoses. Clin Dermatol 2006;24:470–481.
Tarutani M, Imai Y, Yasuda K, et al. Neutrophil-dominant psoriasis-like skin inflammation induced by epidermal-specific expression of Raf in mice. J Dermatol Sci 2010;58:28–35.
Oka M, Berking C, Nesbit M, et al. Interleukin-8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab Invest 2000;80:595–604.
Acknowledgements
We thank Dr U Kikkawa (Kobe University, Japan) for helpful discussions. This study was supported by research grants 19591305 (MO) and 19390296 (CN) from the Scientific Research Funds of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and research grants from Nikkol Group, Cosmos Technical Center, Japan and Rhoto Research Award of Japanese Society of Geriatric Dermatology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Supplementary information
Rights and permissions
About this article
Cite this article
Oka, M., Edamatsu, H., Kunisada, M. et al. Phospholipase Cɛ has a crucial role in ultraviolet B-induced neutrophil-associated skin inflammation by regulating the expression of CXCL1/KC. Lab Invest 91, 711–718 (2011). https://doi.org/10.1038/labinvest.2011.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2011.10
Keywords
This article is cited by
-
Transglutaminase 2 mediates UV-induced skin inflammation by enhancing inflammatory cytokine production
Cell Death & Disease (2017)
-
Knockdown of PLCε inhibits inflammatory cytokine release via STAT3 phosphorylation in human bladder cancer cells
Tumor Biology (2015)
-
UVB-Induced Skin Inflammation and Cutaneous Tissue Injury Is Dependent on the MHC Class I–Like Protein, CD1d
Journal of Investigative Dermatology (2014)
-
Inhibitory Effects of Dietary Spirulina platensis on UVB-Induced Skin Inflammatory Responses and Carcinogenesis
Journal of Investigative Dermatology (2014)
-
Elevated expression patterns and tight correlation of the PLCE1 and NF-κB signaling in Kazakh patients with esophageal carcinoma
Medical Oncology (2014)