Abstract
Objective:
To describe the influence that of l-carnitine supplementation on acylcarnitine (AC) profiles and hospital outcomes in premature infants.
Study Design:
This study is a secondary analysis of previously reported work. Metabolic profiles were obtained using standard newborn techniques on infants born between 23 and 31 completed weeks of gestation. The profiles were drawn within the first 24 h after birth and on approximately days 7, 28 and 42 of life, or at the time of discharge. A single, central, contract laboratory analyzed and managed the samples.
Results:
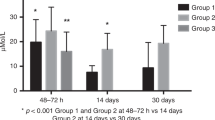
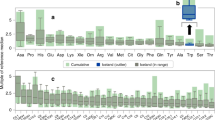
We studied 995 patients; none was subsequently diagnosed with an inborn error of metabolism. l-Carnitine was added to parenteral nutrition in 390 (39%) study subjects; 592 (60%) did not receive supplementation. Non-supplemented infants were more likely to develop low levels of free carnitine (FC; <7 μm) on day 28; (41% vs 5%, P<0.01); and FC values were lower on day 7. Despite higher levels of FC and fewer patients with significant carnitine deficiencies, we found no evidence that l-carnitine supplementation was associated with improved short-term hospital outcomes.
Conclusion:
l-Carnitine supplementation is common in prematurely born neonates and is associated with higher carnitine levels, but is not associated with improved short-term hospital outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Clark RH, Kelleher AS, Chace DH, Spitzer AR . Gestational age and age at sampling influence metabolic profiles in premature infants. Pediatrics 2014; 134: e37–e46.
ElHassan NO, Kaiser JR . Parenteral nutrition in the neonatal intensive care unit. Neoreviews 2011; 12: e130–e140.
Scaglia F. Carnitine deficency. Available at:http://emedicine.medscape.com/article/942233-overview (accessed on 19 February 2016).
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q . Role of carnitine in disease. Nutr Metab 2010; 7: 30.
Rubecz I, Sandor A, Hamar A, Mestyan J . Blood levels of total carnitine and lipid utilization with and without carnitine supplementation in newborn infants. Acta Paediatr Hung 1984; 25: 165–171.
Shenai JP, Borum PR . Tissue carnitine reserves of newborn infants. Pediatr Res 1984; 18: 679–682.
Penn D, Ludwigs B, Schmidt-Sommerfeld E, Pascu F . Effect of nutrition on tissue carnitine concentrations in infants of different gestational ages. Biol Neonate 1985; 47: 130–135.
Van Aerde JE . In preterm infants, does the supplementation of carnitine to parenteral nutrition improve the following clinical outcomes: growth, lipid metabolism and apneic spells?: Part B: clinical commentary. Paediatr Child Health 2004; 9: 573.
Van AT . In preterm infants, does the supplementation of carnitine to parenteral nutrition improve the following clinical outcomes: growth, lipid metabolism and apneic spells?: Part A: evidence-based answer and summary. Paediatr Child Health 2004; 9: 571–572.
Pande S, Brion LP, Campbell DE, Gayle Y, Esteban-Cruciani NV . Lack of effect of L-carnitine supplementation on weight gain in very preterm infants. J Perinatol 2005; 25: 470–477.
Cairns PA, Stalker DJ . Carnitine supplementation of parenterally fed neonates. Cochrane Database Syst Rev 2000; 4: CD000950.
Clark RH, Chace DH, Spitzer AR . Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics 2007; 120: 1286–1296.
Bulbul A, Okan F, Bulbul L, Nuhoglu A . Effect of low versus high early parenteral nutrition on plasma amino acid profiles in very low birth-weight infants. J Matern Fetal Neonatal Med 2012; 25: 770–776.
Yang S, Lee BS, Park HW, Choi YS, Jeong SH, Kim JH et al. Effect of high vs standard early parenteral amino acid supplementation on the growth outcomes in very low birth weight infants. JPEN J Parenter Enteral Nutr 2013; 37: 327–334.
Blanco CL, Gong AK, Green BK, Falck A, Schoolfield J, Liechty EA . Early changes in plasma amino acid concentrations during aggressive nutritional therapy in extremely low birth weight infants. J Pediatr 2011; 158: 543–548.
Blanco CL, Gong AK, Schoolfield J, Green BK, Daniels W, Liechty EA et al. Impact of early and high amino acid supplementation on ELBW infants at 2 years. J Pediatr Gastroenterol Nutr 2012; 54: 601–607.
Vlaardingerbroek H, Vermeulen MJ, Rook D, van den Akker CH, Dorst K, Wattimena JL et al. Safety and efficacy of early parenteral lipid and high-dose amino acid administration to very low birth weight infants. J Pediatr 2013; 163: 638–644.
Chace DH, De Jesus VR, Lim TH, Hannon WH, Clark RH, Spitzer AR . Detection of TPN contamination of dried blood spots used in newborn and metabolic screening and its impact on quantitative measurement of amino acids. Clin Chim Acta 2011; 412: 1385–1390.
Chace DH, Kalas TA, Naylor EW . Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem 2003; 49: 1797–1817.
Fusch C, Bauer K, Böhles HJ, Jochum F, Koletzko B, Krawinkel M et al. Working group for developing the guidelines for parenteral nutrition of The German Society for Nutritional Medicine. Neonatology/Paediatrics-Guidelines on Parenteral Nutrition, Chapter 13. Ger Med Sci 2009; 7: Doc15.(http://www.egms.de/en/gms/2009-7/000074.shtml).
Rebouche CJ, Paulson DJ . Carnitine metabolism and function in humans. Annu Rev Nutr 1986; 6: 41–66.
Marin VB, Azocar M, Molina M, Guerrero JL, Ratner R, Cano F . Total carnitine and acylated carnitine ratio: relationship of free carnitine with lipid parameters in pediatric dialysis patients. Adv Perit Dial 2006; 22: 130–135.
Tim-Aroon T, Harmon HM, Nock ML, Viswanathan SK, McCandless SE . Stopping parenteral nutrition for 3 hours reduces false positives in newborn screening. J Pediatr 2015; 167: 312–316.
Crill CM, Storm MC, Christensen ML, Hankins CT, Bruce JM, Helms RA . Carnitine supplementation in premature neonates: effect on plasma and red blood cell total carnitine concentrations, nutrition parameters and morbidity. Clin Nutr 2006; 25: 886–896.
Acknowledgements
This work was supported by Pediatrix Medical Group. No outside honorarium, grant or other form of payment was given to anyone to produce the manuscript. Study registration number: ClinicalTrials.gov identifier: NCT00865150.
Author contributions
RHC has contributed to the conception and design of the study. The paper from first to final draft was written and edited by RHC with the help of his co-authors who offered suggestions for improvement. DHC has contributed to the conception and design of the study analysis and interpretation of data, as well as the final approval of the version to be published. ARS has contributed to the conception and design of the study, analysis and interpretation of data, critical revisions for important intellectual content, and final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
Clark, R., Chace, D. & Spitzer, A. Impact of l-carnitine supplementation on metabolic profiles in premature infants. J Perinatol 37, 566–571 (2017). https://doi.org/10.1038/jp.2016.253
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.253