Abstract
Objective:
To compare the effectiveness of surfactant delivery via endotracheal tube (ETT) using an intubation-surfactant-rapid extubation approach with premedication) vs laryngeal mask airway (LMA) in preventing the need for mechanical ventilation in preterm neonates with moderate respiratory distress syndrome (RDS).
Study Design:
Moderately preterm infants diagnosed with RDS, receiving nasal continuous positive airway pressure with FiO2 0.30 to 0.60, were randomized to two groups at age 3 to 48 h. Those in the ETT group were intubated following premedication with atropine and morphine, whereas the LMA group received only atropine. Both groups received calfactant before a planned reinstitution of nasal continuous positive airway pressure, and had equivalent pre-specified criteria for subsequent mechanical ventilation and surfactant retreatment. The primary outcome was failure of surfactant treatment strategy to avoid mechanical ventilation; we differentiated early from late failures to assess the contribution of potential mechanisms such as respiratory depression versus less-effective surfactant delivery. Secondary outcomes addressed efficacy and safety end points.
Result:
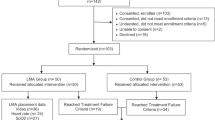
Sixty-one patients were randomized, one excluded and 30 analyzed in each group, with similar baseline characteristics. Failure rate was 77% in the ETT group and 30% in the LMA group (P<0.001). The difference was related to early failure, as late failure rates did not differ between groups. FiO2 decrease after surfactant and rates of adverse events were similar between groups.
Conclusion:
Surfactant therapy through an LMA decreases the proportion of newborns with moderate RDS who require mechanical ventilation, when compared with a standard endotracheal intubation procedure with sedation. The efficacy of surfactant in decreasing RDS severity appears similar with both methods. Morphine premedication likely contributed to early post-surfactant failures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics 1999; 103 (2): E24.
Stevens TP, Harrington EW, Blennow M, Soll RF . Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007; (4): CD003063.
Neonatal Resuscitation Textbook. 6th edn. American Heart Association and American Academy of Pediatrics: Elk Grove Village, IL, USA, 2011.
Dempsey EM, Al Hazzani F, Faucher D, Barrington KJ . Facilitation of neonatal endotracheal intubation with mivacurium and fentanyl in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 2006; 91 (4): F279–F282.
Friesen RH, Honda AT, Thieme RE . Changes in anterior fontanel pressure in preterm neonates during tracheal intubation. Anesth Analg 1987; 66 (9): 874–878.
Marshall TA, Deeder R, Pai S, Berkowitz GP, Austin TL . Physiologic changes associated with endotracheal intubation in preterm infants. Crit Care Med 1984; 12 (6): 501–503.
Barrington K. Canadian Paediatric Society Fetus and Newborn Committee. Premedication for endotracheal intubation in the newborn infant. Paediatr Child Health 2011; 16 (3): 159–171.
Kumar P, Denson SE, Mancuso TJ . American Academy of Pediatrics Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics 2010; 125 (3): 608–615.
Byrne E, MacKinnon R . Should premedication be used for semi-urgent or elective intubation in neonates? Arch Dis Child 2006; 91 (1): 79–83.
Carbajal R, Eble B, Anand KJ . Premedication for tracheal intubation in neonates: confusion or controversy? Semin Perinatol 2007; 31 (5): 309–317.
Kingma PS . Is premedication for intubation of preterm infants the right choice? J Pediatr 2011; 159 (6): 883–884.
Oei J, Hari R, Butha T, Lui K . Facilitation of neonatal nasotracheal intubation with premedication: a randomized controlled trial. J Paediatr Child Health 2002; 38 (2): 146–150.
Bohlin K, Gudmundsdottir T, Katz-Salamon M, Jonsson B, Blennow M . Implementation of surfactant treatment during continuous positive airway pressure. J Perinatol 2007; 27 (7): 422–427.
Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 1994; 331 (16): 1051–1055.
Trevisanuto D, Grazzina N, Ferrarese P, Micaglio M, Verghese C, Zanardo V . Laryngeal mask airway used as a delivery conduit for the administration of surfactant to preterm infants with respiratory distress syndrome. Biol Neonate 2005; 87 (4): 217–220.
Chen L, Hsiao AL . Randomized trial of endotracheal tube versus laryngeal mask airway in simulated prehospital pediatric arrest. Pediatrics 2008; 122 (2): e294–e297.
Brimacombe J, Gandini D, Keller C . The laryngeal mask airway for administration of surfactant in two neonates with respiratory distress syndrome. Paediatr Anaesth 2004; 14 (2): 188–190.
Brimacombe J, Gandini D . Airway rescue and drug delivery in an 800 g neonate with the laryngeal mask airway. Paediatr Anaesth 1999; 9 (2): 178.
Micaglio M, Zanardo V, Ori C, Parotto M, Doglioni N, Trevisanuto D, ProSeal LMA . for surfactant administration. Paediatr Anaesth 2008; 18 (1): 91–92.
Attridge J, Stewart C, Stukenborg G, Kattwinkel J . Administration of rescue surfactant by laryngeal mask airway: lessons from a pilot trial. Am J Perinatol 2013; 30 (3): 201–206.
Roberts KD, Lampland AL, Meyers PA, Worwa CT, Plumm BJ, Mammel MC . Laryngeal mask airway for surfactant administration in a newborn animal model. Pediatr Res 2010; 68 (5): 414–418.
Abdel-Latif ME, Osborn DA . Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev 2011; CD008309.
Verder H, Bohlin K, Kamper J, Lindwall R, Jonsson B . Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatrica 2009; 98 (9): 1400–1408.
Abdi W, Amathieu R, Adhoum A, Poncelet C, Slavov V, Kamoun W et al. Sparing the larynx during gynecological laparoscopy: a randomized trial comparing the LMA Supreme(TM) and the ETT. Acta Anaesthesiol Scand 2010; 54 (2): 141–146.
Zimmert M, Zwirner P, Kruse E, Braun U . Effects on vocal function and incidence of laryngeal disorder when using a laryngeal mask airway in comparison with an endotracheal tube. Eur j Anaesthesiol 1999; 16 (8): 511–515.
Akbar AN, Muzi M, Lopatka CW, Ebert TJ . Neurocirculatory responses to intubation with either an endotracheal tube or a laryngeal mask airway in humans. J Clin Anesth 1996; 8 (3): 194–197.
Blanchard N, Jezraoui P, Milazzo S, Daelman F, Rajaonarivony D, Ossart M . Changes in intraocular pressure with the use of an endotracheal tube or laryngeal mask airway for anesthesia [Variations de la pression intraoculaire selon l'utilisation d'une sonde d'intubation ou d'un masque laryngé en cours d'anesthésie]. Annales Françaises d'Anesthésie et de Réanimation 1996; 15 (7): 1008–1012.
Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res 1997; 42 (3): 348–355.
Bohlin K, Bouhafs RK, Jarstrand C, Curstedt T, Blennow M, Robertson B . Spontaneous breathing or mechanical ventilation alters lung compliance and tissue association of exogenous surfactant in preterm newborn rabbits. Pediatr Res 2005; 57 (5 Pt 1): 624–630.
Dani C, Corsini I, Poggi C . Risk factors for intubation-surfactant-extubation (INSURE) failure and multiple INSURE strategy in preterm infants. Early Hum Dev 2012; 88 (Supplement 1): S3–S4.
Cherif A, Hachani C, Khrouf N . Risk factors of the failure of surfactant treatment by transient intubation during nasal continuous positive airway pressure in preterm infants. Am J Perinatol 2008; 25 (10): 647–652.
Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 2011; 378 (9803): 1627–1634.
Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG . Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 2011; 96 (4): F243–F248.
Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 2013; 98 (2): F122–F126.
van den Berg E, Lemmers PM, Toet MC, Klaessens JH, van BF . Effect of the "InSurE" procedure on cerebral oxygenation and electrical brain activity of the preterm infant. Arch Dis Child Fetal Neonatal Ed 2010; 95 (1): F53–F58.
Welzing L, Kribs A, Huenseler C, Eifinger F, Mehler K, Roth B . Remifentanil for INSURE in preterm infants: a pilot study for evaluation of efficacy and safety aspects. Acta Paediatrica 2009; 98 (9): 1416–1420.
Bahk JH, Sung J, Jang IJ . A comparison of ketamine and lidocaine spray with propofol for the insertion of laryngeal mask airway in children: a double-blinded randomized trial. Anesth Analg 2002; 95 (6): 1586–1589.
Sarkar S, Schumacher RE, Baumgart S, Donn SM . Are newborns receiving premedication before elective intubation? J Perinatol 2006; 26 (5): 286–289.
Whyte S, Birrell G, Wyllie J . Premedication before intubation in UK neonatal units. Arch Dis Child Fetal Neonatal Ed 2000; 82 (1): F38–F41.
Kelleher J, Mallya P, Wyllie J . Premedication before intubation in UK neonatal units: a decade of change? Arch Dis Child Fetal Neonatal Ed 2009; 94 (5): F332–F335.
Eusébio M, Fernandes E . Use of premedication for elective neonatal tracheal intubation in Portugal [O uso de pré-medicação na intubação traqueal não emergente do recém-nascido em Portugal]. Acta Pediatr Port 2008; 39 (1): 3–7.
Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A et al. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics 2012; 129 (4): e952–e959.
Rocha G, Flor-de-Lima F, Proença E, Carvalho C, Quintas C, Martins T et al. Failure of early nasal continuous positive airway pressure in preterm infants of 26 to 30 weeks gestation. J Perinatol 2013; 33 (4): 297–301.
Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U . Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 2013; 131 (2): e502–e509.
Deshpande G, Gill A . Cardiac arrest following naloxone in an extremely preterm neonate. Eur J Pediatr 2009; 168 (1): 115–117.
Falck AJ, Escobedo MB, Baillargeon JG, Villard LG, Gunkel JH . Proficiency of pediatric residents in performing neonatal endotracheal intubation. Pediatrics 2003; 112 (6 Pt 1): 1242–1247.
Acknowledgements
We thank the Neonatology group at the Albany Medical Center NICU, including attending physicians, fellows, nurse practitioners, respiratory therapists and nurses for their cooperation in patient enrollment and protocol adherence. We thank the participating families for their good will and encouragement. We also acknowledge Dr Ashar Ata for agreeing to oversee data safety-monitoring procedures for the study, and Dr James Cummings for his helpful review of the manuscript draft. The study was partly supported by a donation of two LMA Classic devices by LMA North America, to start the project; in addition, ONY, provided an educational grant to the Albany Medical Center, to support Dr Santana-Rivas's research during fellowship. None of the authors received any direct remuneration or salary support for the work. Neither LMA North America, nor ONY, had any involvement in the design, conduct, analysis or publication of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
Pinheiro, J., Santana-Rivas, Q. & Pezzano, C. Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. J Perinatol 36, 196–201 (2016). https://doi.org/10.1038/jp.2015.177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.177
This article is cited by
-
Comparison of laryngeal mask airway and endotracheal tube placement in neonates
Journal of Perinatology (2024)
-
RDS-NExT workshop: consensus statements for the use of surfactant in preterm neonates with RDS
Journal of Perinatology (2023)
-
Should less invasive surfactant administration (LISA) become routine practice in US neonatal units?
Pediatric Research (2023)
-
Current Controversies and Advances in Non-invasive Respiratory Support for Preterm Infants
Current Treatment Options in Pediatrics (2022)
-
Comparison of laryngeal mask airway and endotracheal tube in preterm neonates receiving general anesthesia for inguinal hernia surgery: a retrospective study
BMC Anesthesiology (2021)