Abstract
Objective:
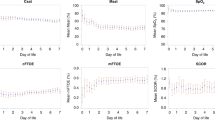
The study investigated the ability of near-infrared spectroscopy (NIRS) to detect subgroups of preterm infants who benefit most from red blood cell (RBC) transfusion in regard to cerebral/renal tissue oxygenation (i) and the number of general oxygen desaturation below 80% (SaO2 <80%) (ii).
Study Design:
Cerebral regional (crSO2) and peripheral regional (prSO2) NIRS parameters were recorded before, during, immediately after and 24 h after transfusion in 76 infants. Simultaneously, SaO2 <80% were recorded by pulse oximetry. To answer the basic question of the study, all preterm infants were divided into two subgroups according to their pretransfusion crSO2 values (<55% and ⩾55%). This cutoff was determined by a k-means clustering analysis.
Result:
crSO2 and prSO2 increased significantly in the whole study population. A stronger increase (P<0.0005) of both was found in the subgroup with pretransfusion crSO2 values <55%. Regarding the whole population, a significant decrease (P<0.05) of episodes with SaO2 <80% was observed. The subgroup with crSO2 baselines <55% had significant (P<0.05) more episodes with SaO2 <80% before transfusion. During and after transfusion, the frequency of episodes with SaO2 <80% decreased more in this group compared with the group with crSO2 baselines ⩾55%.
Conclusion:
NIRS measurement is a simple, non-invasive method to monitor regional tissue oxygenation and the efficacy of RBC transfusion. Infants with low initial NIRS values benefited most from blood transfusions regarding SaO2 <80%, which may be important for their general outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bifano EM, Curran TR . Minimizing donor blood exposure in the neonatal intensive care unit. Current trends and future prospects. Clin Perinatol 1995; 22: 657–669.
Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G . Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 2010; 30: 1220–1226.
Bell EF . When to transfuse preterm babies. Arch Dis Child Fetal Neonatal Ed 2008; 93: 469–473.
Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005; 115: 1685–1691.
Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA et al. The premature infants in need of transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006; 149: 301–307.
Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ . Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol 2004; 24: 763–768.
Stute H, Greiner B, Linderkamp O . Effect of blood transfusion on cardiorespiratory abnormalities in preterm infants. Arch Dis Child Fetal Neonatal Ed 1995; 72: 194–196.
James L, Greenough A, Naik S . The effect of blood transfusion on oxygenation in premature ventilated neonates. Eur J Pediatr 1997; 156: 139–141.
Bailey SM, Hendricks-Munoz KD, Wells JT, Mally P . Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol 2010; 27: 445–453.
Torella F, Cowley R, Thorniley MS, McCollum CN . Monitoring blood loss with near infrared spectroscopy. Comp Biochem Physiol A Mol Integr Physiol 2002; 132: 199–203.
Fuchs H, Lindner W, Buschko A, Almazam M, Hummler HD, Schmid MB . Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatol 2012; 32 (5): 356–362.
Baerts W, Lemmers PM, van Bel F . Cerebral oxygenation and oxygen extraction in the preterm infant during desaturation: effects of increasing FiO(2) to assist recovery. Neonatology 2011; 99 (1): 65–72.
Urlesberger B, Pichler G, Gradnitzer E, Reiterer F, Zobel G, Müller W . Changes in cerebral blood volume and cerebral oxygenation during periodic breathing in term infants. Neuropediatrics 2000; 31 (2): 75–81.
Demirel G, Oguz SS, Celik IH, Erdeve O, Dilmen U . Cerebral and mesenteric tissue oxygenation by positional changes in very low birth weight premature infants. Early Hum Dev 2012; 88 (6): 409–411.
Heldt T, Kashif FM, Sulemanji M, O'Leary HM, du Plessis AJ, Verghese GC . Continuous quantitative monitoring of cerebral oxygen metabolism in neonates by ventilator-gated analysis of NIRS recordings. Acta Neurochir Suppl 2012; 114: 177–180.
van Bel F, Lemmers P, Naulaers G . Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology 2008; 94: 237–244.
Toet MC, Lemmers PM . Brain monitoring in neonates. Early Hum Dev 2009; 85 (2): 77–84.
Marin T, Moore J . Understanding near-infrared spectroscopy. Adv Neonatal Care 2011; 11 (6): 382–388.
Giliberti P, Mondì V, Conforti A, Haywood Lombardi M, Sgrò S, Bozza P et al. Near infrared spectroscopy in newborns with surgical disease. J Matern Fetal Neonatal Med 2011; 24 (Suppl 1): 56–58.
Nicklin SE, Hassan IA, Wickramasinghe YA, Spencer SA . The light still shines, but not that brightly? The current status of perinatal near infrared spectroscopy. Arch Dis Child Fetal Neonatal Ed 2003; 88 (4): F263–F268.
Greisen G, Leung T, Wolf M . Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care? Philos Transact A Math Phys Eng Sci 2011; 369 (1955): 4440–4451.
Van Hoften JC, Verhagen EA, Keating P, ter Horst HJ, Bos AF . Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed 2010; 95: 352–358.
Petrova A, Mehta R . Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr Crit Care 2006; 7: 449–454.
Zaramella P, Freato F, Quaresima V, Secchieri S, Milan A, Grisafi D et al. Early versus late cord clamping: effects on peripheral blood flow and cardiac function in term infants. Early Hum Dev 2008; 84 (3): 195–200.
Baenziger O, Stolkin F, Keel M, von Siebenthal K, Fauchere JC, Das Kundu S et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics 2007; 119 (3): 455–459.
Bailey SM, Hendricks-Muñoz KD, Mally P . Splanchnic-cerebral oxygenation ratio as a marker of preterm infant blood transfusion needs. Transfusion 2012; 52 (2): 252–260.
Henderson-Smart DJ, Steer P . Methylxanthine treatment for apnea in preterm infants. Cochrane Database Syst Rev 2001 (3) CD000140.
Henderson-Smart DJ, De Paoli AG . Prophylactic methylxanthine for prevention of apnoea in preterm infants. Cochrane Database Syst Rev 2010; (12)CD000432.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 2007; 357 (19): 1893–1902.
Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012; 307 (3): 275–282.
Martin RJ, Wilson CG . What to do about apnea of prematurity? J Appl Physiol 2009; 107 (4): 1015–1016.
Zhao J, Gonzalez F, Mu D . Apnea of prematurity: from cause to treatment. Eur J Pediatr 2011; 170 (9): 1097–1105.
Pillekamp F, Hermann C, Keller T, von Gontard A, Kribs A, Roth B . Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology 2007; 91 (3): 155–161.
Urlesberger B, Kaspirek A, Pichler G, Müller W . Apnoea of prematurity and changes in cerebral oxygenation and cerebral blood volume. Neuropediatrics 1999; 30 (1): 29–33.
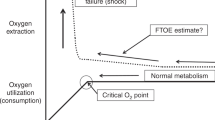
Kissack CM, Garr R, Wardle SP, Weindling AM . Cerebral fractional oxygen extraction is inversely correlated with oxygen delivery in the sick, newborn, preterm infant. J Cereb Blood Flow Metab 2005; 25: 545–553.
Grant PE, Roche-Labarbe N, Surova A, Themelis G, Selb J, Warren EK et al. Increased cerebral blood volume and oxygen consumption in neonatal brain injury. J Cereb Blood Flow Metab 2009; 29 (10): 1704–1713.
McNeill S, Gatenby JC, McElroy S, Engelhardt B . Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol 2011; 31 (1): 51–57.
Roche-Labarbe N, Carp SA, Surova A, Patel M, Boas DA, Grant PE et al. Noninvasive optical measures of CBV, StO(2), CBF index, and rCMRO(2) in human premature neonates' brains in the first six weeks of life. Hum Brain Mapp 2010; 31 (3): 341–352.
Hume H, Blanchette V, Strauss RG, Levy GJ . A survey of Canadian neonatal blood transfusion practices. Transfus Sci 1997; 18: 71–80.
Creteur J, Neves AP, Vincent JL . Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care 2009; 13 (Suppl 5): S11.
Wardle SP, Garr R, Yoxall CW, Weindling AM . A pilot randomised controlled trial of peripheral fractional oxygen extraction to guide blood transfusions in preterm infants. Arch Dis Child Fetal Neonatal Ed 2002; 86: F22–F27.
Bernal NP, Hoffman GM, Ghanayem NS, Arca MJ . Cerebral and somatic near-infrared spectroscopy in normal newborns. J Pediatr Surg 2010; 45: 1306–1310.
Yamamoto A, Yokoyama N, Yonetani M, Uetani Y, Nakamura H, Nakao H . Evaluation of changes of cerebral circulation by SpO2 in preterm infants with apneic episodes using near infrared spectroscopy. Pediatr Int 2003; 45: 661–664.
Delivoria-Papadopoulos M, McGowan J . Oxygen transport and delivery In: Polin R, Fox W (eds). Fetal and Neonatal Physiology 2nd edn. WB Saunders: Philadelphia, 1998 pp 1105–1116.
Leipälä JA, Talme M, Viitala J, Turpeinen U, Fellman V . Blood volume assessment with hemoglobin subtype analysis in preterm infants. Biol Neonate 2003; 84: 41–44.
Hudson I, Cooke A, Holland B, Houston A, Jones JG, Turner T et al. Red cell volume and cardiac output in anaemic preterm infants. Arch Dis Child 1990; 65: 672–675.
Hammerman C . Influence of disease state on oxygen transport in newborn piglets. Biol Neonate 1994; 66: 128–136.
Bauer J, Hentschel R, Linderkamp O . Effect of sepsis syndrome on neonatal oxygen consumption and energy expenditure. Pediatrics 2002; 110: e69.
Booth EA, Dukatz C, Sood BG, Wider M . Near-infrared spectroscopy monitoring of cerebral oxygen during assisted ventilation. Surg Neurol Int 2011; 2: 65.
Ide K, Eliasziw M, Poulin MJ . Relationship between middle cerebral artery blood velocity and end tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol 2003; 95: 129–137.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Seidel, D., Bläser, A., Gebauer, C. et al. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J Perinatol 33, 282–287 (2013). https://doi.org/10.1038/jp.2012.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2012.108
Keywords
This article is cited by
-
The short-term effects of RBC transfusions on intestinal injury in preterm infants
Pediatric Research (2023)
-
Neuromonitoring in neonatal critical care part II: extremely premature infants and critically ill neonates
Pediatric Research (2023)
-
Renal versus cerebral saturation trajectories: the perinatal transition in preterm neonates
Pediatric Research (2022)
-
Cerebral oxygenation in preterm infants receiving transfusion
Pediatric Research (2019)
-
Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions
Pediatric Research (2019)