Abstract
Subtelomeric deletions of 1q44 cause mental retardation, developmental delay and brain anomalies, including abnormalities of the corpus callosum (ACC) and microcephaly in most patients. We report the cases of six patients with 1q44 deletions; two patients with interstitial deletions of 1q44; and four patients with terminal deletions of 1q. One of the patients showed an unbalanced translocation between chromosome 5. All the deletion regions overlapped with previously reported critical regions for ACC, microcephaly and seizures, indicating the recurrent nature of the core phenotypic features of 1q44 deletions. The four patients with terminal deletions of 1q exhibited severe volume loss in the brain as compared with patients who harbored interstitial deletions of 1q44. This indicated that telomeric regions have a role in severe volume loss of the brain. In addition, two patients with terminal deletions of 1q43, beyond the critical region for 1q44 deletion syndrome exhibited delayed myelination. As the deletion regions identified in these patients extended toward centromere, we conclude that the genes responsible for delayed myelination may be located in the neighboring region of 1q43.
Similar content being viewed by others
Introduction
Submicroscopic subtelomeric chromosomal deletions have been found in 7.4% of children with moderate to severe mental retardation.1 Some subtelomeric deletion syndromes are clinically recognizable and identified by characteristic features, whereas some others cannot be identified by such means. The recent development of molecular karyotyping using chromosomal microarray testing has revealed clear genotype–phenotype correlations and identified the critical chromosomal regions of the characteristic features of subtelomeric deletions. The most striking example is the Miller-Diecker syndrome, which has shown clear genotype–phenotype correlations.2 Miller-Diecker syndrome is caused by the subtelomeric deletion of 17p, and is well recognized and characterized by lissencephaly and distinctive facial features,3 which result from the involvement of the platelet-activating factor acetylhydrolase 1b regulatory subunit 1 gene (PAFAH1B1) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase-activation protein epsilon polypeptide gene (YWHAE), respectively; both these genes are located on 17p13.3
Several studies have investigated the critical region for 1q44 subtelomeric deletion syndrome and found that the core phenotypic features of 1q44 subtelomeric deletion syndrome are microcephaly, abnormalities of the corpus callosum (ACC) and seizures.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Recently, Ballif et al.17 analyzed patients with microdeletions of 1q44 and proposed certain genes that may be responsible for individual features.
We report the cases of six newly identified patients with 1q44 deletions; two with interstitial deletion of 1q44; and four with terminal deletion of 1q. As the patients with terminal deletion of 1q44 exhibited more severe phenotypes compared with the patients with interstitial deletion, the phenotypic differences would be derived from additionally deleted region of 1q43q44.
Materials and methods
Subjects
Six Japanese patients were diagnosed as having chromosomal deletions in the region of 1q43q44 in our ongoing study to analyze genomic copy number aberrations. This study was approved by the ethical committee of our institution. After obtaining written consents, we accumulated samples from the patients. Parental samples were also obtained to study their carrier status.
Methods
Genomic copy number was analyzed using the 244, 105 or 60 K Human Genome CGH Microarray (Agilent Technologies, Santa Clara, CA, USA) as described previously.2 Genomic DNAs was extracted from peripheral blood using a standard method. Genomic copy number aberrations were visualized using Agilent Genomic Workbench version 5.5 (Agilent Technologies).
Fluorescence in situ hybridization (FISH) was performed as previously described, when metaphase spreads were available.2 Bacterial artificial clones were selected from the UCSC genome browser (http://www.genome.ucsc.edu). Physical positions refer to the March 2009 human reference sequence. Bacterial artificial clone DNAs were extracted by an automatic DNA extraction system GENE PREP STAR PI-80X (Kurabo, Osaka, Japan).
Results
Chromosomal deletions
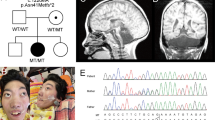
Chromosomal microarray testing revealed aberrations in the 1q43q44 region in six patients (Figure 1). In patient 1 and 2, interstitial deletions of 1.9 and 2.2 Mb, respectively, were identified within the 1q43q44 region. In patient 1, FISH analysis confirmed the deletion (Figure 2a). Subsequent FISH analysis revealed no abnormalities in the parents of both families, indicating de novo deletions in both patients. Molecular karyotyping defined the aberrations as arr 1q44(243 809 193–245 665 521) × 1 dn for patient 1 and arr 1q43q44(243 303 991–245 506 920) × 1 dn for patient 2.
Results of chromosomal microarray testing presented by Gene View of Agilent Genomic Workbench (Agilent Technologies). Vertical axis and horizontal axis represent log2 signal ratio and genomic position, respectively. Aberrant regions are shown by blue rectangles. Dots indicate the genomic positions and the log2 ratio of each probe.
Results of FISH analyses. (a) Loss of the green signal labeling RP11-7L23 (arrow) indicates deletion of 1q44 in patient 1. (b) Loss of the green signal labeling RP11-88N11 (arrow) indicates deletion of 1q44 in patient 3. (c–e) Confirmation of the unbalanced translocation between chromosome 1 and 5 in patient 4. Loss of the green signal labeling RP11-143E8 (arrow) indicates deletion of 1q44 (c). An additional signal labeling RP11-94J21 of 5p15.33 is present on the other chromosome (d, red signal, arrow), indicating a translocation onto chromosome 1 (e, green signal, arrow).
In patient 3, terminal deletions of 1q43 were identified and confirmed by FISH analyses (Figure 2b). As FISH analyses for both parents showed no abnormalities, this deletion occurred as de novo. Molecular karyotyping of patient 3 was indicated as arr 1q43q44(242 442 098–249 250 621) × 1 dn.
In patient 4, a loss of genomic copy number at 1q43q44 and an additional gain at 5p15.33 were identified (Supplementary Figure 1). Subsequent FISH analysis confirmed an unbalanced translocation between 1q43 and 5p in patient 4 (Figures 2c–e), and no translocation was found in either parents. Consequently, the patient’s unbalance translocation was determined to be de novo in origin. Her karyotype was 46,XX,der(1)t(1;5)(q43;p15.33).arr 1q43q44(242 223 230–249 212 668) × 1, 5p15.33(57 640–1 705 515) × 3 dn. The duplicated region of 5p was only 1.7 Mb of the terminal region.
In patient 5, terminal deletions of 1q43 were identified with a breakpoint in the v-akt murine thymoma viral oncogene homolog 3 gene (AKT3). The molecular karyotype was arr 1q44(243 880 099–249 212 668) × 1. The largest deletion was identified in patient 6 with arr 1q43q44(238 888 870–249 212 668) × 1.
The result of FISH analysis were summarized in Supplementary Table 1.
Clinical report
Patient 1
A 9-year-old boy was born by vacuum extraction. His birth weight was 2820 g (within 25th centile), length was 45 cm (=3rd centile) and occipitofrontal circumference (OFC) was 32.5 cm (10–25th centile). After birth, he displayed feeding problem. His development was mildly delayed with head control attained at 8 months, sitting without support at 15 months, crawling at 24 months and walking alone without support at 44 months. At the age of 11 months, he experienced recurrent febrile seizures. At 18 months of age, he suffered non-febrile seizures. The brain magnetic resonance imaging (MRI) at 4 years of age revealed loss of volume in the frontal lobe, mild abnormal gyral patterns in the frontal lobe and lateral lobe, and ACC (Figures 3a–c). Conventional chromosomal analysis revealed a normal male karyotype.
The brain MRI findings of patient 1 examined at 4 years (a–c), patient 2 examined at 3 years (d–f), patient 3 examined at 12 months (g–i), patient 4 examined at 15 months (j–l), patient 5 examined at 5 months (m–o) and patient 6 examined at 3 years (p–r). T1-weighted sagittal views (a, d, g, j, m, p), T1-weighted axial views (b, e, h, k, n, q) and T2-weighted axial views (c, f, i, l, o, r). ACC are noted in all patients. Patient 5 (m), in particular, exhibits complete agenesis of the corpus callosum. Reduced volume of the frontal lobe is seen in all patients. Prominently delayed myelination is noted in patient 3 and 4 (i and l).
At present, his height is 119.5 cm (<3rd centile) and weight is 23.6 kg (10th centile). He exhibits microcephaly with OFC of 47.5 cm (<3rd centile). He displays distinctive facial features including a flat occipit, coarse face, high nasal bridge, low-set ears and prominent jaw (Figure 4a). He has short tapering fingers and single palmer creases.
Patient 2
A 3-year and 1-month-old girl is a second child of healthy non-consanguineous parents with an unremarkable family history. She was born at 36th week of gestation after an uneventful pregnancy. She displayed prenatal growth retardation with her weight of 2348 g (<3rd centile), length of 44.4 cm (<3rd centile) and OFC of 31 cm (<3rd centile). At the age of 6 months, developmental delay and microcephaly were noted. She could balance her head at 6 months, sit at 10 months, roll over at 13 months and crawl at 20 months. At the age of 11 months, she suffered febrile convulsion, followed by 11 recurrent attacks of complex febrile seizures. The findings of her electroencephalogram were unremarkable.
At the age of 2 years and 11 months, she had a short stature (height 79 cm, <3rd centile; weight 10 kg, <3rd centile) and microcephaly (OFC 42.5 cm, <3rd centile). Facial dysmorphism included epicanthal folds, a broad nasal root and down-turned corner of mouth (Figure 4b). Neurological examination revealed generalized hypotonia. At this time, she showed moderate psychomotor developmental delay with motor development of 10 months and language development of 8 months. Ultrasonography of the kidneys and liver, echocardiography, ophthalmologic and audiologic examinations revealed no abnormalities. Conventional chromosome analysis revealed a normal female karyotype.
At present, the patient cannot stand without support. She can speak only babbled words. The brain MRI revealed ACC and loss of the volume of the frontal lobe (Figures 3d–f).
Patient 3
A 1-year and 6-month-old girl was born at the gestational age of 38 weeks by vaginal delivery. Although her pregnancy was unremarkable, she showed prenatal growth retardation with her birth weight of 2040, g (<3rd centile) and OFC of 30 cm (<3rd centile). Her APGAR score was 9/9. One hour after birth, she suffered a convulsion triggered by hypoglycemia. Ultrasonography showed enlarged bilateral cerebral ventricles and intraventricular hemorrhage. Echocardiography showed no abnormalities. Ophthalmologic examination displayed exotropia and atrophy of the right retina and optic papilla. Auditory brain-stem response (ABR) showed normal results. Owing to her feeding difficulty, she required tube feeding. At the age of 5 months, she experienced a status convulsive epilepticus, and recurrent spike and waves were noted on EEG at that time. She was treated with several antiepileptic drugs. The brain MRI examined at 1 year of age showed ACC and reduced volume of the brain (Figures 3g–i). Low intensity of the white matter was only noted on the posterior limb of internal capsule, indicating delayed myelination (Figure 3i).
At 1 year and 6 months, the patient could not control her head by herself. She did not demonstrate eye contact, and her feeding difficulties persisted. Distinct facial features included sparse hair, microcephaly, a flat occipit, a coarse face, a high nasal bridge, low-set ears, micrognathia, inversion of eyelids, esotropia and atopic skin. Immediately after this examination, acute respiratory infectious disease caused hypoxic brain damages, which was confirmed by brain computed tomography that revealed the presence of multi-cystic lesions (data not shown). After this event, she shows spastic quadriplegia.
Patient 4
A 3-year and 3-month-old girl was born by induced delivery at 37th week as a first child of healthy, non-consanguineous parents. She showed prenatal growth retardation with her birth weight of 2386 g (<3rd centile), length of 44 cm (<3rd centile) and OFC 31 cm (<3rd centile). Atrial septal defect was revealed by ultrasonography. She displayed psychomotor developmental delay with holding up her head at 5 months, sitting by herself at 14 months and crawling at 20 months. From the age of 14 months, she was prescribed with antiepileptic drugs because of status convulsive that continued for ∼1 h. The brain MRI examined at an age of 15 months revealed ACC and hypoplastic brain (Figures 3j–l). Low intensity of the white matter was only noted in the posterior limb of the internal capsule, indicating delayed myelination (Figure 3l). Conventional karyotyping on metaphase spreads prepared from peripheral lymphocytes showed a normal female karyotype.
At present, the patient exhibits severe developmental delay as she cannot stand without support and her language usage is limited to babbled words. Her mental and psychomotor developmental index of the Bayley Scales of Infant Development (II) indicate 6 and 8 months, respectively. She demonstrates growth deficiency and microcephaly with her weight of 12.8 kg (25–50th centile), height of 87.7 cm (10th centile) and OFC of 44 cm (<3rd centile). Dysmorphic features include hypertelorism, low-set ears and micrognathia.
Patient 5
A 6-year-old girl was born at 38 weeks of gestation, with birth weight of 1870 g (<3rd centile), length of 42 cm (<3rd centile) and OFC of 28 cm (<3rd centile), indicating intrauterine growth restriction. Since early infancy, she exhibited severe hypotonia, and her psychomotor development was severely delayed. She experienced refractory seizures after 1 year of age. Although hydronephrosis was noted by abdominal ultrasonography, there was no abnormal renal function. The brain MRI examined at an age of 4 months revealed a hypoplastic brain associated with complete agenesis of the corpus callosum (Figures 3m–o). As a result of recurrent aspiration pneumonia, laryngotracheal separation surgery was performed. At present, her height is 99 cm (<3rd centile), weight is 15.3 kg (<3rd centile) and OFC of 42 cm (<3rd centile), indicating severe growth deficiency and microcephaly. She can stand by support, but no meaningful words.
Patient 6
A 10-year-old girl was born at 40 weeks and 3 days of gestation. At the age of 3 years, she was referred to our institution because of intractable epilepsy. At that time, neurological examination revealed generalized hypotonia. EEG showed frequent spikes on the left hemisphere. The brain MRI indicated a hypoplastic brain associated with ACC and delayed myelination (Figures 3p–r). Echocardiography revealed mild aortic regurgitation. At present, her height is 115 cm (<3rd centile), weight is 16.3 kg (<3rd centile) and OFC of 48 cm (<3rd centile), indicating growth delay and microcephaly. She shows severe psychomotor developmental delay with sitting by support and no meaningful word.
Discussion
The characteristics of 1q44 deletions have been recognized as growth deficiency, psychomotor developmental delay, epilepsy, microcephaly, brain malformations including ACC, and distinct facial features.4, 6, 7, 9, 11, 18 After chromosomal microarray testing has been available for the identification of submicroscopic chromosomal aberrations, many patients with submicroscopic deletions of 1q44 have been identified.8, 10, 12, 14, 15 Now, precise genotype–phenotype correlation has been evaluated through the accumulation of patients with variable deletion sizes, and a minimal essential region has been proposed for expressing the main characteristics of 1q44 deletion syndrome.7, 8, 14, 15, 16 Ballif et al.17 evaluated 22 patients with small interstitial deletions of 1q44 and demonstrated critical regions for microcephaly, ACC and seizures. Consequently, AKT3 was reported to be the gene responsible for microcephaly; zinc finger protein 238 gene (ZNF238) for ACC; and heterogeneous nuclear ribonucleoprotein U gene (HNRNPU) for seizures17 (Figure 5).
Deletions regions of the present patients depicted on a genome map from UCSC Genome Browser. Blue rectangles indicate the deletion region of the interstitial deletions identified on patients 1 and 2. Blue trapezoids indicate the terminal deletions identified on patients 3–6. Directions of the pointed trapezoids indicate continuous deletions to the telomeres. The region responsible for microcephaly, ACC and seizure that were proposed by Ballif et al.17 are shown by gray rectangles.
In this study, we identified six patients with the deletions including 1q44. Clinical and genetic findings were summarized in Table 1 and Figure 5, respectively. The presenting patient 1 and 2 showed typical interstitial deletions of 1q44, in which AKT3 and ZNF238 are included, and manifested the typical features of mental retardation, microcephaly, ACC and seizures. This evidence supports the findings reported by Ballif et al. that the essential features of 1q44 deletion syndrome are derived from deletions of the minimal region including AKT3 and ZNF238.17 In comparison with these two patients, the other four patients displayed terminal deletion of 1q44 that include the minimal region including AKT3 and ZNF238. Although the chromosomal breakpoint of patient 5 was just on AKT3, phenotypic feature of this patient are extremely severe as compared with those of patient 1 and 2 who had interstitial deletion of 1q44. Although Roos et al.18 reported that 1.6 Mb terminal region does not have clinical relevance, because it appears to primarily contain many of the olfactory receptors genes, many genes of unknown functions are included in this 2.6 Mb region (chr1:245 000 000–247 600 000). Thus, this additionally deleted region in patient 5 may also have been responsible for his severe neurological manifestations.
Patient 4 had an additional chromosomal aberration, with a partial trisomy of 5p derived from an unbalanced translocation. However, the phenotypic severity of patient 4 was not significantly different from that of the other patients with 1q43 deletions. Thus, the partial trisomy of 5p identified in patient 4 did not demonstrate an important contribution to the patient’s manifestations. Patient 3 showed extremely severe developmental delay, which may have been modified by the hypoglycemia and subsequent intraventricular hemorrhage suffered during the early infantile period.
The deletion regions of the remaining three patients (patient 3, 4 and 6) expanded toward the centromere beyond the critical region for 1q44 deletion syndrome, and several more genes are included in the deletion region in these three patients. Severe volume loss of the brain was revealed in the brain MRI of these three patients, similar to patient 5, and delayed myelination was also seen on the brain MRI of patient 3 and 4 (Figures 3i). Although patient 5 exhibited high T2 signal intensities in the white matter, this image was obtained when he was 4 months of age, and we cannot ascertain if this finding indicated delayed myelination. Similarly, because the brain MRI of patient 1, 2 and 6 were obtained after 3 years of age, we cannot determine whether these three patients experienced delayed myelinations in their early infancy. However, delayed myelination, as a finding associated with 1q44 deletion, has not yet been reported in the literature.17 It may therefore be that the characteristic findings of the patients with expanded deletion beyond the 1q43 region may have clinical implications for delayed myelination. Although many UCSC genes are present in these additional regions, some of these genes may have clinical relevance for delayed myelination observed in patient 3 and 4. The centrosomal protein 170 kDa gene (CEP170) is a potential candidate because of its high expression level in the fetal brain.
In this genotype–phenotype correlation study for patients with 1q44 deletions, we revealed that telemetric region beyond the physical position of chr1:245 000 000 may be responsible for severe volume loss of the brain, and the proximal region beyond AKT3 may be responsible for delayed myelination. The neighboring genes surrounding 1q43q44 may have some modifier effects to the severe brain impairments associated with delayed myelination. To identify them, much more information regarding genotype–phenotype correlations will need to be accumulated for patients with terminal deletion of 1q.
References
Knight, S. J., Regan, R., Nicod, A., Horsley, S. W., Kearney, L., Homfray, T. et al. Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 354, 1676–1681 (1999).
Shimojima, K., Sugiura, C., Takahashi, H., Ikegami, M., Takahashi, Y., Ohno, K. et al. Genomic copy number variations at 17p13.3 and epileptogenesis. Epilepsy Res. 89, 303–309 (2010).
Cardoso, C., Leventer, R. J., Ward, H. L., Toyo-Oka, K., Chung, J., Gross, A. et al. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome, and other phenotypes secondary to deletions of 17p13.3. Am. J. Hum. Genet. 72, 918–930 (2003).
Gentile, M., Di Carlo, A., Volpe, P., Pansini, A., Nanna, P., Valenzano, M. C. et al. FISH and cytogenetic characterization of a terminal chromosome 1q deletion: clinical case report and phenotypic implications. Am. J. Med. Genet. A 117A, 251–254 (2003).
Puthuran, M. J., Rowland-Hill, C. A., Simpson, J., Pairaudeau, P. W., Mabbott, J. L., Morris, S. M. et al. Chromosome 1q42 deletion and agenesis of the corpus callosum. Am. J. Med. Genet. A 138, 68–69 (2005).
van Bever, Y., Rooms, L., Laridon, A., Reyniers, E., van Luijk, R., Scheers, S. et al. Clinical report of a pure subtelomeric 1qter deletion in a boy with mental retardation and multiple anomalies adds further evidence for a specific phenotype. Am. J. Med. Genet. A 135, 91–95 (2005).
Boland, E., Clayton-Smith, J., Woo, V. G., McKee, S., Manson, F. D., Medne, L. et al. Mapping of deletion and translocation breakpoints in 1q44 implicates the serine/threonine kinase AKT3 in postnatal microcephaly and agenesis of the corpus callosum. Am. J. Hum. Genet. 81, 292–303 (2007).
Hill, A. D., Chang, B. S., Hill, R. S., Garraway, L. A., Bodell, A., Sellers, W. R. et al. A 2-Mb critical region implicated in the microcephaly associated with terminal 1q deletion syndrome. Am. J. Med. Genet. A 143A, 1692–1698 (2007).
Merritt, J. L., Zou, Y., Jalal, S. M., Michels, V. V. Delineation of the cryptic 1qter deletion phenotype. Am. J. Med. Genet. A 143, 599–603 (2007).
Andrieux, J., Cuvellier, J. C., Duban-Bedu, B., Joriot-Chekaf, S., Dieux-Coeslier, A., Manouvrier-Hanu, S. et al. A 6.9 Mb 1qter deletion/4.4 Mb 18pter duplication in a boy with extreme microcephaly with simplified gyral pattern, vermis hypoplasia and corpus callosum agenesis. Eur. J. Med. Genet. 51, 87–91 (2008).
Hiraki, Y., Okamoto, N., Ida, T., Nakata, Y., Kamada, M., Kanemura, Y. et al. Two new cases of pure 1q terminal deletion presenting with brain malformations. Am. J. Med. Genet. A 146A, 1241–1247 (2008).
Poot, M., Kroes, H. Y., Hochstenbach, R. AKT3 as a candidate gene for corpus callosum anomalies in patients with 1q44 deletions. Eur. J. Med. Genet. 51, 689–690 (2008).
van Bon, B. W., Koolen, D. A., Borgatti, R., Magee, A., Garcia-Minaur, S., Rooms, L. et al. Clinical and molecular characteristics of 1qter microdeletion syndrome: delineating a critical region for corpus callosum agenesis/hypogenesis. J. Med. Genet. 45, 346–354 (2008).
Orellana, C., Rosello, M., Monfort, S., Oltra, S., Quiroga, R., Ferrer, I. et al. Corpus callosum abnormalities and the controversy about the candidate genes located in 1q44. Cytogenet. Genome Res. 127, 5–8 (2009).
Caliebe, A., Kroes, H. Y., van der Smagt, J. J., Martin-Subero, J. I., Tonnies, H., van’t Slot, R. et al. Four patients with speech delay, seizures and variable corpus callosum thickness sharing a 0.440 Mb deletion in region 1q44 containing the HNRPU gene. Eur. J. Med. Genet 53, 179–185 (2010).
Nagamani, S. C., Erez, A., Bay, C., Pettigrew, A., Lalani, S. R., Herman, K. et al. Delineation of a deletion region critical for corpus callosal abnormalities in chromosome 1q43-q44. Eur. J. Hum. Genet. 20, 176–179 (2012).
Ballif, B. C., Rosenfeld, J. A., Traylor, R., Theisen, A., Bader, P. I., Ladda, R. L. et al. High-resolution array CGH defines critical regions and candidate genes for microcephaly, abnormalities of the corpus callosum, and seizure phenotypes in patients with microdeletions of 1q43q44. Hum. Genet. 131, 145–156 (2012).
Roos, A., Eggermann, T., Zerres, K., Schuler, H. M. Polymorphic subtelomeric deletion 1q demonstrates the need to reevaluate subtelomere screening methods: determination of the boundary between pathogenic deletion and benign variant for subtelomere 1q. Am. J. Med. Genet. A 146A, 795–798 (2008).
Acknowledgements
We thank the patients and their parents for their gracious participation and support. This work was supported by Grant-in-Aid for Research Activity Start-up for KS and Grant-in-Aid for Scientific Research (C) for TY from the Japan Ministry of Education, Science, Sports and Culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Shimojima, K., Okamoto, N., Suzuki, Y. et al. Subtelomeric deletions of 1q43q44 and severe brain impairment associated with delayed myelination. J Hum Genet 57, 593–600 (2012). https://doi.org/10.1038/jhg.2012.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2012.77
Keywords
This article is cited by
-
Maternal interchromosomal insertional translocation leading to 1q43-q44 deletion and duplication in two siblings
Molecular Cytogenetics (2018)
-
Infantile spasms related to a 5q31.2-q31.3 microdeletion including PURA
Human Genome Variation (2018)
-
Characteristics of rare and private deletions identified in phenotypically normal individuals
Human Genome Variation (2017)
-
Contribution of copy number variants (CNVs) to congenital, unexplained intellectual and developmental disabilities in Lebanese patients
Molecular Cytogenetics (2015)
-
Molecular karyotyping by array CGH in a Russian cohort of children with intellectual disability, autism, epilepsy and congenital anomalies
Molecular Cytogenetics (2012)