Abstract
Context:
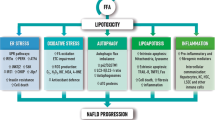
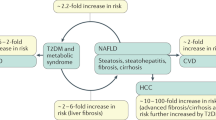
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome and is strongly associated with obesity, dyslipidaemia and altered glucose regulation. Previous data demonstrated that low circulating levels of tumour necrosis factor weak inducer of apoptosis (sTWEAK) were associated with obesity, diabetes and insulin resistance, all traits associated with an increased risk of NALFD. Circulating sTWEAK levels are expected to be reduced in the presence of NAFLD.
Objective:
We aimed to explore the relationship between NAFLD and circulating sTWEAK levels in obese patients, and to evaluate the effect of sTWEAK on hepatocyte triglyceride accumulation.
Design setting and patients:
This is an observational case–control study performed in n=112 severely obese patients evaluated for NAFLD by abdominal ultrasound and n=32 non-obese patients without steatosis. Serum sTWEAK concentrations were measured by ELISA. Multivariable analyses were performed to determine the independent predictors of NAFLD. We analysed TWEAK and Fn14 protein expression in liver biopsies by western blotting and immunohistochemistry. An immortalized primary human hepatocyte cell line (HHL) was used to evaluate the effect of sTWEAK on triglyceride accumulation.
Results:
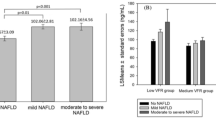
We observed a reduction in serum circulating sTWEAK concentrations with the presence of liver steatosis. On multivariable analysis, lower sTWEAK concentrations were independently associated with the presence of NAFLD (odds ratio (OR)=0.023; 95% confidence interval: 0.001–0.579; P<0.022). In human hepatocytes, sTWEAK administration reduced fat accumulation as demonstrated by the reduction in palmitic acid-induced accumulation of triglyceride and the decreased expression of cluster of differentiation 36 (CD36) and perilipin 1 and 2 (PLIN1 and PLIN2) genes.
Conclusions:
Decreased sTWEAK concentrations are independently associated with the presence of NAFLD. This is concordant with the observation that TWEAK reduces lipid accumulation in human liver cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Browning JD, Horton JD . Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004; 114: 147–152.
Farrell GC, Larter CZ . Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006; 43: S99–S112.
Perry RJ, Samuel VT, Petersen KF, Shulman GI . The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014; 510: 84–91.
Winkles JA . The TWEAK-Fn1 4 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 2008; 7: 411–425.
Moreno JA, Muñoz-Garcí a B, Martín-Ventura JL, Madrigal-Matute J, Orbe J, Páramo JA et al. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis 2009; 207: 103–110.
Maymó-Masip E, Fernández-Veledo S, Garcia Españ a A, Vázquez-Carballo A, Tinahones FJ, García-Fuentes E et al. The rise of soluble TWEAK levels in severely obese subjects after bariatric surgery may affect adipocyte-cytokine production induced by TNFα. J Clin Endocrinol Metab 2013; 98: E1323–E1333.
Vázquez-Carballo A, Ceperuelo-Mallafré V, Chacón MR, Maymó-Masip E, Lorenzo M, Porras et al. TWEAK prevents TNF-α-induced insulin resistance through PP2A activation in human adipocytes. Am J Physiol Endocrinol Metab 2013; 305: E101–E112.
Vendrell J, Chacón MR . TWEAK: a new player in obesity and diabetes. Front Immunol 2013; 4: 488.
Simón-Muela I, Llauradó G, Chacón MR, Olona M, Näf S, Maymó-Masip E et al. Reduced circulating levels of TWEAK are associated with gestational diabetes mellitus. Eur J Clin Invest 2015; 45: 27–35.
Llauradó G, González-Clemente J-M, Maymó-Masip E, Subías D, Vendrell J, Chacón MR . Serum levels of TWEAK and scavenger receptor CD163 in type 1 diabetes mellitus: relationship with cardiovascular risk factors. a case-control study. PLoS One 2012; 7: e43919.
Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lössner U, Blüher M et al. Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 2008; 199: 440–444.
Díaz-López A, Chacón MR, Bulló M, Maymó-Masip E, Martínez-González MA, Estruch R et al. Serum sTWEAK concentrations and risk of developing type 2 diabetes in a high cardiovascular risk population: a nested case-control study. J Clin Endocrinol Metab 2013; 98: 3482–3490.
Díaz-López A, Bulló M, Chacón MR, Estruch R, Vendrell J, Díez-Espino J et al. Reduced circulating sTWEAK levels are associated with metabolic syndrome in elderly individuals at high cardiovascular risk. Cardiovasc Diabetol 2014; 13: 51.
Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest 2005; 115: 2330–2340.
Dwyer BJ, Olynyk JK, Ramm GA, Tirnitz-Parker JE . TWEAK and LTβ signaling during chronic liver disease. Front Immunol 2014; 5: 39.
Kazantzis M, Stahl A . Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta 2012; 1821: 852–857.
Glatz JFC, Luiken JJFP, Bonen A . Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 2010; 90: 367–417.
Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SLM, Han X-X et al. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc 2004; 63: 245–249.
Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 2008; 134: 556–567.
Semple RK, Chatterjee VKK, Rahilly SO . Review series PPARγ and human metabolic disease. J Clin Invest 2006; 116: 581–589.
Lee JH, Zhou J, Xie W . PXR and LXR in hepatic steatosis: a new dog and an old dog with new tricks. Mol Pharm 2008; 5: 60–66.
Strauss S, Gavish E, Gottlieb P, Katsnelson L . Interobserver and intraobserver variability in the sonographic assessment of fatty liver. Am J Roentgenol 2007; 189: 1449.
Brunt EM . Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001; 21: 3–16.
Chacón MR, Richart C, Gómez JM, Megí a A, Vilarrasa N, Fernández-Real JM et al. Expression of TWEAK and its receptor Fn14 in human subcutaneous adipose tissue. Relationship with other inflammatory cytokines in obesity. Cytokine 2006; 33: 129–137.
Vendrell J, Maymó-Masip E, Tinahones F, García-Españ a A, Megia A, Caubet E et al. Tumor necrosis-like weak inducer of apoptosis as a proinflammatory cytokine in human adipocyte cells: up-regulation in severe obesity is mediated by inflammation but not hypoxia. J Clin Endocrinol Metab 2010; 95: 2983–2992.
Clayton RF, Rinaldi A, Kandyba EE, Edward M, Willberg C, Klenerman P et al. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int 2005; 25: 389–402.
Willberg CB, Ward SM, Clayton RF, Naoumov NV, McCormick C, Proto S et al. Protection of hepatocytes from cytotoxic T cell mediated killing by interferon-alpha. PLoS One 2007; 2: e791.
Luo Y, Rana P, Will Y . Cyclosporine A and palmitic acid treatment synergistically induce cytotoxicity in HepG2 cells. Toxicol Appl Pharmacol 2012; 261: 172–180.
Olagnier D, Lavergne R-A, Meunier E, Lefèvre L, Dardenne C, Aubouy et al. Nrf2, a PPARγ alternative pathway to promote CD36 expression on inflammatory macrophages: implication for malaria. PLoS Pathog 2011; 7: e1002254.
Hong C, Walczak R, Dhamko H, Bradley MN, Marathe C, Boyadjian R et al. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J Lipid Res 2011; 52: 531–539.
Larsen TM, Toubro S, Astrup A . PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord 2003; 27: 147–161.
Yki-Järvinen H . Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2: 901–910.
Kazankov K, Tordjman J, Møller HJ, Vilstrup H, Poitou C, Bedossa P et al. Macrophage activation marker soluble CD163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J Gastroenterol Hepatol 2015; 30: 1293–1300.
Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 2007; 46: 823–830.
Shafritz DA . To TWEAK, or not to TWEAK: that is the question. Hepatology 2010; 52: 13–15.
Fausto N . Tweaking liver progenitor cells. Nat Med 2005; 11: 1053–1054.
Steneberg P, Sykaras AG, Backlund F, Straseviciene J, Soderstrom I, Edlund H . Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem 2015; 290: 19034–19043.
Koonen DPY, Jacobs L, Febbraio M, Young ME, Soltys CM . Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007; 56: 2863–2871.
Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011; 60: 1394–1402.
Carr RM, Peralta G, Yin X, Ahima RS . Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One 2014; 9: e97118.
Okumura T . Role of lipid droplet proteins in liver steatosis. J Physiol Biochem 2011; 67: 629–636.
Zhao Y-P, Li L, Ma J-P, Chen G, Bai J-H . LXRα gene downregulation by lentiviral-based RNA interference enhances liver function after fatty liver transplantation in rats. Hepatobiliary Pancreat Dis Int 2015; 14: 386–393.
Labrie M, Lalonde S, Najyb O, Thiery M, Daneault C, Des Rosiers C et al. Apolipoprotein D transgenic mice develop hepatic steatosis through activation of PPARγ and fatty acid uptake. PLoS One 2015; 10: e0130230.
Bohte AE, Van Werven JR, Bipat S, Stoker J . The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 2011; 21: 87–97.
Acknowledgements
This study was supported by projects from the Fondo de Investigación Sanitaria (FIS): PI 11/00049 (MRC), PI14/00465 (MRC) and by CB07/08/0012 from CIBERDEM (Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas). All projects from FIS are co-financed by the European Regional Development Fund (ERDF). Dr MR Chacón is supported by the Research Stabilization Programme of the Instituto de Salud Carlos III (ISCIII) co-financed by Institut Català de Salut (ICS) in Catalonia. Dr Rodriguez is financed by a fellowship 'Juan de la Cierva' from the Spanish Ministry of Science and Innovation (JCI-2011-11488). Dr Llauradó was financed by a fellowship 'Rio Hortega' from the Instituto de Salud Carlos III (ISCIII).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Lozano-Bartolomé, J., Llauradó, G., Rodriguez, M. et al. Reduced circulating levels of sTWEAK are associated with NAFLD and may affect hepatocyte triglyceride accumulation. Int J Obes 40, 1337–1345 (2016). https://doi.org/10.1038/ijo.2016.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.73