Abstract
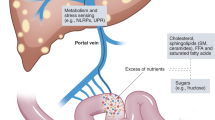
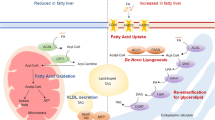
The increasing epidemic of obesity worldwide is linked to serious health effects, including increased prevalence of type 2 diabetes mellitus, cardiovascular disease and nonalcoholic fatty liver disease (NAFLD). NAFLD is the liver manifestation of the metabolic syndrome and includes the spectrum of liver steatosis (known as nonalcoholic fatty liver) and steatohepatitis (known as nonalcoholic steatohepatitis), which can evolve into progressive liver fibrosis and eventually cause cirrhosis. Although NAFLD is becoming the number one cause of chronic liver diseases, it is part of a systemic disease that affects many other parts of the body, including adipose tissue, pancreatic β-cells and the cardiovascular system. The pathomechanism of NAFLD is multifactorial across a spectrum of metabolic derangements and changes in the host microbiome that trigger low-grade inflammation in the liver and other organs. Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear regulatory factors that provide fine tuning for key elements of glucose and fat metabolism and regulate inflammatory cell activation and fibrotic processes. This Review summarizes and discusses the current literature on NAFLD as the liver manifestation of the systemic metabolic syndrome and focuses on the role of PPARs in the pathomechanisms as well as in the potential targeting of disease.
Key points
-
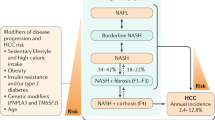
Nonalcoholic steatohepatitis (NASH) is the fastest growing liver disease worldwide; however, it is often not recognized until advanced disease stages.
-
The management and treatment of NASH, the liver manifestation of the metabolic syndrome, require a holistic approach.
-
Peroxisome proliferator-activated receptors (PPARs) regulate metabolism, inflammation and fibrosis, all of which determine NASH progression.
-
There is an urgent need for medical therapy for patients with NASH.
-
Both PPARα-β/δ dual agonism as well as PPARγ agonism have shown beneficial effects on liver histology in phase IIb clinical trials for NASH.
-
Single, dual and pan-PPAR agonists are under development for the pharmacological treatment of NASH.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease — Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
European Association for the Study of the Liver, European Association for the Study of Diabetes & European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Angulo, P., Machado, M. V. & Diehl, A. M. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin. Liver Dis. 35, 132–145 (2015).
Cholankeril, G. et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig. Dis. Sci. 62, 2915–2922 (2017).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018). An important modelling approach emphasizing the global trends in increasing prevalence of NAFLD and its related morbidity and mortality.
Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol. 62 (Suppl. 1), S47–S64 (2015). This review cites the evidence that NAFLD has consequences beyond the liver and specifically increases the risk of T2DM.
Francque, S. M., van der Graaff, D. & Kwanten, W. J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 65, 425–443 (2016). This review summarizes the mechanisms that link NAFLD to CVD.
Tilg, H., Moschen, A. R. & Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 14, 32–42 (2017).
Lallukka, S. & Yki-Jarvinen, H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 30, 385–395 (2016).
Adams, L. A., Anstee, Q. M., Tilg, H. & Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 66, 1138–1153 (2017).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71, 793–801 (2019).
Targher, G. & Byrne, C. D. A perspective on metabolic syndrome and nonalcoholic fatty liver disease. Metab. Syndr. Relat. Disord. 13, 235–238 (2015).
Francque, S. et al. High prevalence of advanced fibrosis in association with the metabolic syndrome in a Belgian prospective cohort of NAFLD patients with elevated ALT. Results of the Belgian NAFLD registry. Acta Gastroenterol. Belg. 74, 9–16 (2011).
Gastaldelli, A. & Cusi, K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 1, 312–328 (2019). This review describes the crucial role of dysfunctional adipose tissue in the close relationship between diabetes and NAFLD.
Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910 (2014).
Wainwright, P. & Byrne, C. D. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int. J. Mol. Sci. 17, 367 (2016).
Mantovani, A., Byrne, C. D., Bonora, E. & Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 41, 372–382 (2018).
Targher, G., Byrne, C. D., Lonardo, A., Zoppini, G. & Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J. Hepatol. 65, 589–600 (2016). This meta-analysis cites the evidence that NAFLD is an independent risk factor for incident cardiovascular events.
Sattar, N. et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 139, 2228–2237 (2019).
Millett, E. R. C., Peters, S. A. E. & Woodward, M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ 363, k4247 (2018).
Stepanova, M., Rafiq, N. & Younossi, Z. M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut 59, 1410–1415 (2010).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155 (2015).
Tada, T. et al. Type 2 diabetes mellitus: a risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 34, 2011–2018 (2019).
Yang, J. D. et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology 71, 907–916 (2020).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397.e10 (2015).
Musso, G., Cassader, M., Paschetta, E. & Gambino, R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern. Med. 177, 633–640 (2017).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Francque, S. & Vonghia, L. Pharmacological treatment for non-alcoholic fatty liver disease. Adv. Ther. 36, 1052–1074 (2019).
Konerman, M. A., Jones, J. C. & Harrison, S. A. Pharmacotherapy for NASH: current and emerging. J. Hepatol. 68, 362–375 (2018).
Derosa, G., Sahebkar, A. & Maffioli, P. The role of various peroxisome proliferator-activated receptors and their ligands in clinical practice. J. Cell. Physiol. 233, 153–161 (2018).
Targher, G., Lonardo, A. & Byrne, C. D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 14, 99–114 (2018).
Haas, J. T., Francque, S. & Staels, B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol. 78, 181–205 (2016).
Mann, J. P., Valenti, L., Scorletti, E., Byrne, C. D. & Nobili, V. Nonalcoholic fatty liver disease in children. Semin. Liver Dis. 38, 1–13 (2018).
Fleet, S. E., Lefkowitch, J. H. & Lavine, J. E. Current concepts in pediatric nonalcoholic fatty liver disease. Gastroenterol. Clin. North. Am. 46, 217–231 (2017).
Newton, K. P. et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 170, e161971 (2016).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Rinella, M. E., Tacke, F., Sanyal, A. J., Anstee, Q. M. & Participants of the AASLD/EASL Workshop. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology 70, 1424–1436 (2019).
Samuel, V. T. & Shulman, G. I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 27, 22–41 (2018).
Gancheva, S., Jelenik, T., Alvarez-Hernandez, E. & Roden, M. Interorgan metabolic crosstalk in human insulin resistance. Physiol. Rev. 98, 1371–1415 (2018).
Jacome-Sosa, M. M. & Parks, E. J. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr. Opin. Lipidol. 25, 213–220 (2014).
Apostolopoulou, M. et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 41, 1235–1243 (2018).
Bril, F. et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 65, 1132–1144 (2017).
Dai, W. et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine 96, e8179 (2017).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 (2017).
Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 142, 711–725.e6 (2012). Reviews the key role of adipose tissue and lipotoxicity in the development of muscle and liver insulin resistance and metabolic syndrome and the rationale for PPARγ insulin sensitizers in NASH.
Diehl, A. M. & Day, C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N. Engl. J. Med. 377, 2063–2072 (2017).
Neuschwander-Tetri, B. A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52, 774–788 (2010).
Bessone, F., Razori, M. V. & Roma, M. G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol. Life Sci. 76, 99–128 (2019).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018).
Tamura, S. & Shimomura, I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1139–1142 (2005).
Roden, M. & Shulman, G. I. The integrative biology of type 2 diabetes. Nature 576, 51–60 (2019). This review summarizes the earliest events leading to insulin resistance, ectopic fat deposition and hyperglycaemia in humans and points to the decisive role of dysfunctional adipose tissue.
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005). A classic work describing the contribution of adipose tissue to hepatic steatosis and liver insulin resistance in NAFLD.
Bril, F. & Cusi, K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 40, 419–430 (2017).
Liss, K. H. & Finck, B. N. PPARs and nonalcoholic fatty liver disease. Biochimie 136, 65–74 (2017).
Barb, D., Portillo-Sanchez, P. & Cusi, K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism 65, 1183–1195 (2016).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 (2017).
Jindal, A. et al. Fat-laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH). Exp. Mol. Pathol. 99, 155–162 (2015).
Zhou, Z. et al. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol. Gastroenterol. Hepatol. 5, 399–413 (2018).
Grunhut, J. et al. Macrophages in nonalcoholic steatohepatitis: friend or foe? Eur. Med. J. Hepatol. 6, 100–109 (2018).
Szabo, G. & Csak, T. Role of microRNAs in NAFLD/NASH. Dig. Dis. Sci. 61, 1314–1324 (2016).
Szabo, G. & Csak, T. Inflammasomes in liver diseases. J. Hepatol. 57, 642–654 (2012). Reviews the role of inflammasome activation in chronic inflammation associated with fibrosis and cirrhosis in liver diseases.
Ganz, M. et al. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 13, 193 (2015). Describes the development of a murine model consisting of a high fat–cholesterol–sugar diet that mimics liver pathology associated with NAFLD progression in humans and characterizes sterile and microbial danger signals associated with inflammation linked to NAFLD disease progression.
Chu, H., Williams, B. & Schnabl, B. Gut microbiota, fatty liver disease, and hepatocellular carcinoma. Liver Res. 2, 43–51 (2018).
Jayakumar, S. & Loomba, R. Review article: emerging role of the gut microbiome in the progression of nonalcoholic fatty liver disease and potential therapeutic implications. Aliment. Pharmacol. Ther. 50, 144–158 (2019).
Marra, F. & Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 68, 280–295 (2018).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Krenkel, O. et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut 69, 551–563 (2020). This single-cell RNA sequencing analysis of NASH mouse models revealed a striking heterogeneity of myeloid cells and a unique inflammatory polarization of macrophages in NAFLD.
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019). Reviews the role of inflammatory macrophages in disease severity of NASH and highlights studies of potential treatments for patients with NASH that target macrophage recruitment and polarization.
Lefere, S. et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J. Hepatol. 73, 757–770 (2020).
Dreyer, C. et al. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68, 879–887 (1992).
Issemann, I. & Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347, 645–650 (1990).
Wanders, R. J. & Waterham, H. R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 75, 295–332 (2006).
Michalik, L. et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58, 726–741 (2006).
Fajas, L. et al. The organization, promoter analysis, and expression of the human PPARγ gene. J. Biol. Chem. 272, 18779–18789 (1997).
Tailleux, A., Wouters, K. & Staels, B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim. Biophys. Acta 1821, 809–818 (2012).
Rakhshandehroo, M., Hooiveld, G., Muller, M. & Kersten, S. Comparative analysis of gene regulation by the transcription factor PPARα between mouse and human. PLoS ONE 4, e6796 (2009).
de la Rosa Rodriguez, M. A. et al. The whole transcriptome effects of the PPARα agonist fenofibrate on livers of hepatocyte humanized mice. BMC Genomics 19, 443 (2018). This paper shows the differences between humans and mice in terms of PPARα activity and target genes.
Roberts, R. A. et al. Apoptosis and proliferation in nongenotoxic carcinogenesis: species differences and role of PPARα. Toxicol. Lett. 112–113, 49–57 (2000).
Holden, P. R. & Tugwood, J. D. Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J. Mol. Endocrinol. 22, 1–8 (1999).
Kersten, S. & Stienstra, R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 136, 75–84 (2017).
Cheung, C. et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res. 64, 3849–3854 (2004).
Bell, A. R. et al. Molecular basis of non-responsiveness to peroxisome proliferators: the guinea-pig PPARα is functional and mediates peroxisome proliferator-induced hypolipidaemia. Biochem. J. 332, 689–693 (1998).
Lawrence, J. W. et al. Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) alpha. PPAR alpha fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expresson. J. Biol. Chem. 276, 31521–31527 (2001).
Pap, A., Cuaranta-Monroy, I., Peloquin, M. & Nagy, L. Is the mouse a good model of human PPARgamma-related metabolic diseases? Int. J. Mol. Sci. 17, 1236 (2016).
Su, A. I. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl Acad. Sci. USA 101, 6062–6067 (2004).
Vidal-Puig, A. et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Invest. 97, 2553–2561 (1996).
Francque, S. et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 63, 164–173 (2015).
Kim, S. M. et al. Novel PPARα agonist MHY553 alleviates hepatic steatosis by increasing fatty acid oxidation and decreasing inflammation during aging. Oncotarget 8, 46273–46285 (2017).
Chakravarthy, M. V. et al. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 138, 476–488 (2009).
Reid, B. N. et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 283, 13087–13099 (2008).
Xu, H. E. et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc. Natl Acad. Sci. USA 98, 13919–13924 (2001).
Braissant, O., Foufelle, F., Scotto, C., Dauca, M. & Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137, 354–366 (1996).
Montagner, A. et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 65, 1202–1214 (2016).
Lefebvre, P., Chinetti, G., Fruchart, J. C. & Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Invest. 116, 571–580 (2006).
Zardi, E. M. et al. Hepatic PPARs: their role in liver physiology, fibrosis and treatment. Curr. Med. Chem. 20, 3370–3396 (2013).
Chen, L. et al. Oleoylethanolamide, an endogenous PPAR-alpha ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oncotarget 6, 42530–42540 (2015).
Wang, Z. et al. Taurine protected As2O3-induced the activation of hepatic stellate cells through inhibiting PPARα-autophagy pathway. Chem. Biol. Interact. 300, 123–130 (2019).
Tardelli, M., Claudel, T., Bruschi, F. V., Moreno-Viedma, V. & Trauner, M. Adiponectin regulates AQP3 via PPARα in human hepatic stellate cells. Biochem. Biophys. Res. Commun. 490, 51–54 (2017).
Bougarne, N. et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 39, 760–802 (2018).
Pawlak, M. et al. The transrepressive activity of peroxisome proliferator-activated receptor alpha is necessary and sufficient to prevent liver fibrosis in mice. Hepatology 60, 1593–1606 (2014).
Kersten, S. et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103, 1489–1498 (1999).
Sanderson, L. M., Boekschoten, M. V., Desvergne, B., Muller, M. & Kersten, S. Transcriptional profiling reveals divergent roles of PPARalpha and PPARbeta/delta in regulation of gene expression in mouse liver. Physiol. Genomics 41, 42–52 (2010).
Lemberger, T. et al. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J. Biol. Chem. 271, 1764–1769 (1996).
Canaple, L. et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol. Endocrinol. 20, 1715–1727 (2006).
Guan, D. et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell 174, 831–842.e12 (2018).
Tognini, P. et al. Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab. 26, 523–538.e5 (2017).
Gachon, F. et al. Proline- and acidic amino acid-rich basic leucine zipper proteins modulate peroxisome proliferator-activated receptor alpha (PPARalpha) activity. Proc. Natl Acad. Sci. USA 108, 4794–4799 (2011).
Botta, M. et al. PPAR agonists and metabolic syndrome: an established role? Int. J. Mol. Sci. 19, 1197 (2018).
Pawlak, M., Lefebvre, P. & Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 62, 720–733 (2015).
Liu, S. et al. Role of peroxisome proliferator-activated receptor δ/β in hepatic metabolic regulation. J. Biol. Chem. 286, 1237–1247 (2011).
Liu, S. et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 502, 550–554 (2013).
Iwaisako, K. et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc. Natl Acad. Sci. USA 109, E1369–E1376 (2012).
Dietz, M. et al. Comparative molecular profiling of the PPARα/γ activator aleglitazar: PPAR selectivity, activity and interaction with cofactors. ChemMedChem 7, 1101–1111 (2012).
Ricote, M. & Glass, C. K. PPARs and molecular mechanisms of transrepression. Biochim. Biophys. Acta 1771, 926–935 (2007).
Zizzo, G. & Cohen, P. L. The PPAR-γ antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: a key role for PPAR-γ in human macrophage polarization. J. Inflamm. 12, 36 (2015).
Wilding, J. P. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes. Metab. 14, 973–982 (2012).
Han, L., Shen, W. J., Bittner, S., Kraemer, F. B. & Azhar, S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 13, 259–278 (2017).
Han, L., Shen, W. J., Bittner, S., Kraemer, F. B. & Azhar, S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 13, 279–296 (2017).
Delerive, P. et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 274, 32048–32054 (1999).
Hou, X. & Pei, F. Estradiol inhibits cytokine-induced expression of VCAM-1 and ICAM-1 in cultured human endothelial cells via AMPK/PPARα activation. Cell Biochem. Biophys. 72, 709–717 (2015).
Hoekstra, M., Kruijt, J. K., Van Eck, M. & Van Berkel, T. J. Specific gene expression of ATP-binding cassette transporters and nuclear hormone receptors in rat liver parenchymal, endothelial, and Kupffer cells. J. Biol. Chem. 278, 25448–25453 (2003).
Girroir, E. E. et al. Quantitative expression patterns of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) protein in mice. Biochem. Biophys. Res. Commun. 371, 456–461 (2008).
Auboeuf, D. et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes 46, 1319–1327 (1997).
Fan, Y. et al. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 315–321 (2008).
Kilgore, K. S. & Billin, A. N. PPARbeta/delta ligands as modulators of the inflammatory response. Curr. Opin. Investig. Drugs 9, 463–469 (2008).
Liu, Y. et al. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int. J. Mol. Sci. 19, 3339 (2018).
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 (2008).
Lanthier, N. et al. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G107–G116 (2010).
Dulai, P. S. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 65, 1557–1565 (2017).
Hagstrom, H. et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273 (2017).
Weiskirchen, R., Weiskirchen, S. & Tacke, F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 65, 2–15 (2019).
Lefere, S. & Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 1, 30–43 (2019). This is an elegant review on the role of macrophages in NAFLD.
Ritz, T., Krenkel, O. & Tacke, F. Dynamic plasticity of macrophage functions in diseased liver. Cell Immunol. 330, 175–182 (2018).
Ham, S. A. et al. Ligand-activated PPARδ upregulates α-smooth muscle actin expression in human dermal fibroblasts: a potential role for PPARδ in wound healing. J. Dermatol. Sci. 80, 186–195 (2015).
Park, J. R. et al. Effects of peroxisome proliferator-activated receptor-δ agonist on cardiac healing after myocardial infarction. PLoS ONE 11, e0148510 (2016).
Lefebvre, P. et al. Interspecies NASH disease activity whole-genome profiling identifies a fibrogenic role of PPARα-regulated dermatopontin. JCI Insight 2, e92264 (2017).
Kato, A. et al. Identification of fibronectin binding sites in dermatopontin and their biological function. J. Dermatol. Sci. 76, 51–59 (2014).
Soccio, R. E., Chen, E. R. & Lazar, M. A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 20, 573–591 (2014).
Ma, X., Wang, D., Zhao, W. & Xu, L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front. Endocrinol. 9, 473 (2018).
Lumeng, C. & Saltiel, A. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Byrne, C. D. & Targher, G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 34, 1155–1161 (2014).
Belfort, R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355, 2297–2307 (2006).
Cusi, K. et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann. Intern. Med. 165, 305–315 (2016). Long-term 3-year study confirming the efficacy of pioglitazone for the treatment of NASH in patients with prediabetes or T2DM.
Aithal, G. P. et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135, 1176–1184 (2008).
Lomonaco, R. et al. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care 39, 632–638 (2016). A study that dissects the relative contribution of adipose tissue, hepatic and muscle insulin resistance in patients with and without diabetes and simple steatosis versus NASH.
Larter, C. Z. et al. Peroxisome proliferator-activated receptor-alpha agonist, Wy 14,643, improves metabolic indices, steatosis and ballooning in diabetic mice with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 27, 341–350 (2012).
Belfort, R., Berria, R., Cornell, J. & Cusi, K. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J. Clin. Endocrinol. Metab. 95, 829–836 (2010).
Fabbrini, E. et al. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 95, 2727–2735 (2010).
Palmer, C. N., Hsu, M. H., Griffin, K. J., Raucy, J. L. & Johnson, E. F. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol. Pharmacol. 53, 14–22 (1998).
Fruchart, J. C. et al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMα) paradigm: conceptual framework and therapeutic potential: a consensus statement from the International Atherosclerosis Society (IAS) and the Residual Risk Reduction Initiative (R3i) Foundation. Cardiovasc. Diabetol. 18, 71 (2019).
Basaranoglu, M., Acbay, O. & Sonsuz, A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J. Hepatol. 31, 384 (1999).
Honda, Y. et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci. Rep. 7, 42477 (2017).
Araki, E. et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52-week data from the PROVIDE study. Diabetes Obes. Metab. 21, 1737–1744 (2019).
Yokote, K. et al. Long-term efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor-alpha modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int. J. Mol. Sci. 20, 706 (2019).
Maeda, N. et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099 (2001).
Gastaldelli, A. et al. Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment. Pharmacol. Ther. 32, 769–775 (2010).
Ratziu, V. et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135, 100–110 (2008).
Ratziu, V. et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51, 445–453 (2010).
Leclercq, I. A., Sempoux, C., Starkel, P. & Horsmans, Y. Limited therapeutic efficacy of pioglitazone on progression of hepatic fibrosis in rats. Gut 55, 1020–1029 (2006).
Bril, F. et al. Role of oral vitamin E for the treatment of nonalcoholic steatohepatitis (NASH) in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 42, 1481–1488 (2019).
Sakamoto, J. et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem. Biophys. Res. Commun. 278, 704–711 (2000).
Kalavalapalli, S. et al. Pioglitazone improves hepatic mitochondrial function in a mouse model of nonalcoholic steatohepatitis. Am. J. Physiol. Endocrinol. Metab. 315, E163–E173 (2018).
Ahmadian, M. et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Devchand, P. R., Liu, T., Altman, R. B., FitzGerald, G. A. & Schadt, E. E. The pioglitazone trek via human PPAR gamma: From discovery to a medicine at the FDA and beyond. Front. Pharmacol. 9, 1093 (2018).
Jain, M. R. et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 38, 1084–1094 (2018).
Kaul, U. et al. New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Cardiovasc. Diabetol. 18, 80 (2019).
Zydus. Zydus announces regulatory filing of Saroglitazar Magnesium for treatment of NASH with DCGI. Zydus Cadila https://zyduscadila.com/public/pdf/pressrelease/Zydus_announces_NDA_filing_of_Saroglitazar_Magnesium_with_DCGI_for_treatment_of_NASH.pdf (2019).
Hong, F., Xu, P. & Zhai, Y. The opportunities and challenges of peroxisome proliferator-activated receptors ligands in clinical drug discovery and development. Int. J. Mol. Sci. 19, 2189 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03061721 (2019).
Haczeyni, F. et al. The selective peroxisome proliferator-activated receptor-delta agonist seladelpar reverses nonalcoholic steatohepatitis pathology by abrogating lipotoxicity in diabetic obese mice. Hepatol. Commun. 1, 663–674 (2017).
Bays HE, E. A. MBX-8025, a novel peroxisome proliferator receptor-delta agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. J. Clin. Endocrinol. Metab. 96, 2889–2897 (2011).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03551522 (2019).
CymaBay Therapeutics. CymaBay Therapeutics reports topline 12-week data from an ongoing phase 2b study of seladelpar in patients with nonalcoholic steatohepatitis. CymaBay https://ir.cymabay.com/press-releases?year=2019&page=2 (2019).
CymaBay Therapeutics. CymaBay Therapeutics halts clinical development of seladelpar. CymaBay https://ir.cymabay.com/press-releases?year=2019&page=1 (2019).
Staels, B. et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 58, 1941–1952 (2013).
Ratziu, V. et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150, 1147–1159.e5 (2016).
Cariou, B. et al. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 36, 2923–2930 (2013).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02704403 (2020).
GENFIT. GENFIT: Announces results from interim analysis of RESOLVE-IT phase 3 trial of elafibranor in adults with NASH and fibrosis. GENFIT https://ir.genfit.com/news-releases/news-release-details/genfit-announces-results-interim-analysis-resolve-it-phase-3 (2020).
McVicker, B. L. & Bennett, R. G. Novel anti-fibrotic therapies. Front. Pharmacol. 8, 318 (2017).
Vallee, A., Vallee, J. N. & Lecarpentier, Y. Metabolic reprogramming in atherosclerosis: Opposed interplay between the canonical WNT/beta-catenin pathway and PPARgamma. J. Mol. Cell Cardiol. 133, 36–46 (2019).
Zhao, N. et al. Enhanced MiR-711 transcription by PPARγ induces endoplasmic reticulum stress-mediated apoptosis targeting calnexin in rat cardiomyocytes after myocardial infarction. J. Mol. Cell Cardiol. 118, 36–45 (2018).
Peymani, M., Ghaedi, K., Irani, S. & Nasr-Esfahani, M. H. Peroxisome proliferator-activated receptor gamma activity is required for appropriate cardiomyocyte differentiation. Cell J. 18, 221–228 (2016).
Ortiz-Lopez, C. et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 35, 873–878 (2012).
DeFronzo, R. A. et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364, 1104–1115 (2011).
Inzucchi, S. E. et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care 39, 1684–1692 (2016).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2006).
Chiquette, E., Ramirez, G. & Defronzo, R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch. Intern. Med. 164, 2097–2104 (2004).
Goldberg, R. B. et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, 1547–1554 (2005).
Mazzone, T. et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296, 2572–2581 (2006).
Nissen, S. E. et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299, 1561–1573 (2008).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 (2005). Paradigm-changing study on the ability of a diabetes medication and insulin-sensitizer (pioglitazone) to reduce stroke and myocardial infarction in patients with T2DM.
Lincoff, A. M., Wolski, K., Nicholls, S. J. & Nissen, S. E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298, 1180–1188 (2007).
Kernan, W. N. et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374, 1321–1331 (2016). Landmark study on the ability of pioglitazone to reduce risk of stroke or myocardial infarction compared with placebo in patients with insulin resistance but without diabetes with a recent history of ischaemic stroke or transient ischaemic attack.
Spence, J. D. et al. Pioglitazone therapy in patients with stroke and prediabetes: a post hoc analysis of the IRIS randomized clinical trial. JAMA Neurol. 76, 526–535 (2019).
Nissen, S. E. & Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 (2007).
Hoogwerf, B. J. et al. Perspectives on some controversies in cardiovascular disease risk assessment in the pharmaceutical development of glucose-lowering medications. Diabetes Care 39, S219–S227 (2016).
Home, P. D. et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373, 2125–2135 (2009). This study, which enrolled more than 4,000 patients, found that rosiglitazone does not increase the risk of overall cardiovascular morbidity or mortality compared with other glucose-lowering drugs.
US Food and Drug Administration. FDA Drug Safety Communication: FDA eliminates the Risk Evaluation and Mitigation Strategy (REMS) for rosiglitazone-containing diabetes medicines (FDA, 2015).
Choi, Y. J. et al. Effects of the PPAR-delta agonist MBX-8025 on atherogenic dyslipidemia. Atherosclerosis 220, 470–476 (2012).
Keech, A. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366, 1849–1861 (2005). This large study found that fenofibrate statistically significantly reduces total cardiovascular events and primarily non-fatal myocardial infarctions.
Ginsberg, H. N. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574 (2010).
Jani, R. H. et al. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol. Ther. 16, 63–71 (2014).
Wettstein, G. et al. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol. Commun. 1, 524–537 (2017).
Boubia, B. et al. Design, synthesis, and evaluation of a novel series of indole sulfonamide peroxisome proliferator activated receptor (PPAR) alpha/gamma/delta triple activators: discovery of lanifibranor, a new antifibrotic clinical candidate. J. Med. Chem. 61, 2246–2265 (2018).
Ruzehaji, N. et al. Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann. Rheum. Dis. 75, 2175–2183 (2016).
Avouac, J. et al. Pan-PPAR agonist IVA337 is effective in experimental lung fibrosis and pulmonary hypertension. Ann. Rheum. Dis. 76, 1931–1940 (2017).
Stumvoll, M. & Haring, H. U. Glitazones: clinical effects and molecular mechanisms. Ann. Med. 34, 217–224 (2002).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03008070 (2020).
Inventiva. Inventiva’s lanifibranor meets the primary and key secondary endpoints in the Phase IIb NATIVE clinical trial in non-alcoholic steatohepatitis (NASH). Inventiva https://inventivapharma.com/inventivas-lanifibranor-meets-the-primary-and-key-secondary-endpoints-in-the-phase-iib-native-clinical-trial-in-non-alcoholic-steatohepatitis-nash/ (2020).
Bonds, D. E. et al. Fenofibrate-associated changes in renal function and relationship to clinical outcomes among individuals with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) experience. Diabetologia 55, 1641–1650 (2012).
Davis, T. M. et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 54, 280–290 (2011).
Lee, M., Saver, J. L., Liao, H. W., Lin, C. H. & Ovbiagele, B. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis. Stroke 48, 388–393 (2017).
DeFronzo, R. A., Inzucchi, S., Abdul-Ghani, M. & Nissen, S. E. Pioglitazone: the forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab. Vasc. Dis. Res. 16, 133–143 (2019).
Portillo-Sanchez, P. et al. Effect of pioglitazone on bone mineral density in patients with nonalcoholic steatohepatitis: a 36-month clinical trial. J. Diab. 11, 223–231 (2019).
Filipova, E., Uzunova, K., Kalinov, K. & Vekov, T. Pioglitazone and the risk of bladder cancer: a meta-analysis. Diabetes Ther. 8, 705–726 (2017).
Balas, B. et al. Pioglitazone treatment increases whole body fat but not total body water in patients with non-alcoholic steatohepatitis. J. Hepatol. 47, 565–570 (2007).
Young, L. H. et al. Heart failure after ischemic stroke or transient ischemic attack in insulin-resistant patients without diabetes mellitus treated with pioglitazone. Circulation 138, 1210–1220 (2018). This secondary analysis of the IRIS trial found that pioglitazone did not increase the risk of heart failure.
van der Meer, R. W. et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 119, 2069–2077 (2009).
Clarke, G. D. et al. Pioglitazone improves left ventricular diastolic function in subjects with diabetes. Diabetes Care 40, 1530–1536 (2017).
Lehrke, M. & Marx, N. Diabetes mellitus and heart failure. Am. J. Cardiol. 120, S37–S47 (2017).
DeFronzo, R. A. et al. Revitalization of pioglitazone: the optimum agent to be combined with a sodium-glucose co-transporter-2 inhibitor. Diabetes Obes. Metab. 18, 454–462 (2016).
Munigoti, S. P. & Harinarayan, C. V. Role of glitazars in atherogenic dyslipidemia and diabetes: two birds with one stone? Indian J. Endocrinol. Metab. 18, 283–287 (2014).
Hirschfield, G. et al. LBP-002 — Treatment efficacy and safety of seladelpar, a selective peroxisome proliferator-activated receptor delta agonist, in primary biliary cholangitis patients: 12- and 26-week analysis from an ongoing international, randomized, dose raging phase 2 study. J. Hepatol. 68, S105–S106 (2018).
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus (WHO, 1999).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Alberti, K. G., Zimmet, P., Shaw, J. & IDF Epidemiology Task Force Consensus Group. The metabolic syndrome — a new worldwide definition. Lancet 366, 1059–1062 (2005).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and international association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03350165 (2019).
Bril, F. et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 42, 1481–1488 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03459079 (2020).
Sumida, Y. & Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 53, 362–376 (2018).
Author information
Authors and Affiliations
Contributions
G.S. and S.F. researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed/edited the manuscript before submission. M.F.A., C.D.B., K.C., J.-F.D., M.R., F.S. and F.T. made a substantial contribution to the discussion of content and reviewed/edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
S.F. has a senior clinical research mandate from the Fund for Scientific Research (FWO) Flanders (1802154N) and has acted as advisor and/or lecturer for Roche, Gilead, Abbvie, Bayer, BMS, MSD, Janssen, Actelion, Astellas, Genfit, Inventiva, Intercept, Genentech and Galmed. G.S. has received research support from NIAAA (NIH), Gilead, Intercept, Allergan, Genfit, Novartis, SignaBlock, Shire, the University of Florida, BMS, Genentech, Takeda, and Vertex. She is a consultant/advisory board member for Allergan, Arrow Diagnostics, Pandion Therapeutics, Glympse Bio, Quest Diagnostic, Surrozen, Innovate Biopharmaceuticals, Alnylam, Zomagen, Novartis, Durect, Generon and Terrafirma. She is an author for UptoDate, editor for the American Association for the Study of Liver Diseases and Editor-in-Chief of Hepatology Communications. M.F.A. is supported by the National Institute of Health (NIH)/NIDDK Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN, U01DK061713, PI: A.M. Diehl) and is an advisor/consultant for Bristol Myers Squibb, NGM Pharma, Inventiva, Taiwan J, Immuron, Prometheus, and Novo-Nordisk. Her institution receives funding for research from NIH/NIDDK, Inventiva, Enyo, Enanta, Allergan, Novartis, Genfit, Intercept, BMS, NGM Parma, Gilead, Conatus, Durect, Poxel, Madrigal, Celgene, Galactin, Galmed, Novo-Nordisk, Taiwan J, Prometheus, TARGET NASH, and Progenity. She serves on speaker’s bureau for Simply Speaking NASH, iHEP NASH, PRIME NASH Programming, Clinical Care Options, and Alexion. C.D.B. is supported by the National Institute for Health Research (NIHR) through the NIHR Southampton Biomedical Research Centre. He is a consultant for Inventiva. K.C. has received research support for the University of Florida as principal investigator from the NIH, Cirius, Echosens, Inventiva, Novartis, Novo Nordisk, Poxel, TARGET NASH and Zydus. He is a consultant for Allergan, Astra-Zeneca, BMS, Boehringer Ingelheim, Coherus, Eli Lilly, Genentech, Gilead, Janssen, Merck, Pfizer, Poxel, Prosciento, Novo Nordisk, Sanofi-Aventis, and TARGET NASH. J.-F.D. is a consultant/advisory board member for Abbvie, Allergan, Bayer, Bristol-Myers Squibb, Falk, Genfit, Genkyotex, Gilead Science, HepaRegenix, Intercept Pharma, Lilly, Merck, and Novartis. He serves as an investigator of studies supported by Abbvie, Bayer, BMS, Falk, Genfit, Gilead Science, Intercept, Inventiva, Lilly, Merck, and Novartis. M.R. has received research support from the Ministry of Culture and Science of the State of North Rhine-Westphalia and the German Federal Ministry of Health, grants from the European Fonds for Regional Development (EFRE-0400191), German Research Foundation (DFG, SFB 1116/2) and the Schmutzler Stiftung, serves as investigator of studies supported by Boehringer-Ingelheim Pharma, Nutricia/Danone, and Sanofi, and was advisor/consultant for Bristol-Myers Squibb, Eli Lilly, Gilead, Intercept Pharma, Novo Nordisk, Novartis, Poxel, Prosciento, Sanofi, Servier, and TARGET NASH. F.S. is a consultant to Pfizer, AstraZeneca, and Abbvie. F.T. has received research funding at Charité University Medicine Berlin from Allergan, Bristol-Myers Squibb, Galapagos, and Inventiva. He is a consultant for Allergan, Bayer, Boehringer Ingelheim, Galapagos, Galmed, Intercept, Inventiva, and Pfizer.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Francque, S., Szabo, G., Abdelmalek, M.F. et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol 18, 24–39 (2021). https://doi.org/10.1038/s41575-020-00366-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-00366-5
This article is cited by
-
Association of non-alcoholic fatty liver disease with self-reported osteoarthritis among the US adults
Arthritis Research & Therapy (2024)
-
Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes
Scientific Reports (2024)
-
Immunology of gut microbiome and liver in non-alcoholic fatty liver disease (NAFLD): mechanisms, bacteria, and novel therapeutic targets
Archives of Microbiology (2024)
-
The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review
European Journal of Clinical Pharmacology (2024)
-
Sub-nanosized vanadate hybrid clusters maintain glucose homeostasis and restore treatment response in inflammatory disease in obese mice
Nano Research (2024)