Abstract
Background:
Considerable evidence suggests that the time of day at which calories are consumed markedly impacts body weight gain and adiposity. However, a precise quantification of energy balance parameters during controlled animal studies enforcing time-of-day-restricted feeding is currently lacking in the absence of direct human interaction.
Objective:
The purpose of the present study was therefore to quantify the effects of restricted feeding during the light (sleep)-phase in a fully-automated, computer-controlled comprehensive laboratory animal monitoring system (CLAMS) designed to modulate food access in a time-of-day-dependent manner. Energy balance, gene expression (within metabolically relevant tissues), humoral factors and body weight were assessed.
Results:
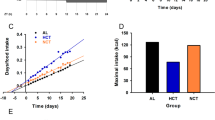
We report that relative to mice fed only during the dark (active)-phase, light (sleep)-phase fed mice: (1) consume a large meal upon initiation of food availability; (2) consume greater total calories per day; (3) exhibit a higher respiratory exchange ratio (indicative of decreased reliance on lipid/fatty acid oxidation); (4) exhibit tissue-specific alterations in the phases and amplitudes of circadian clock and metabolic genes in metabolically active tissues (greatest phase differences observed in the liver and diminution of amplitudes in epididymal fat, gastrocnemius muscle and heart); (5) exhibit diminished amplitude in humoral factor diurnal variations (for example, corticosterone); and (6) exhibit greater weight gain within 9 days of restricted feeding.
Conclusions:
Collectively, these data suggest that weight gain following light (sleep)-phase restricted feeding is associated with significant alterations in energy balance, as well as dyssynchrony between metabolically active organs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Froy O . Metabolism and circadian rhythms—implications for obesity. Endocr Rev 2010; 31: 1–24.
Giovannini M, Verduci E, Scaglioni S, Salvatici E, Bonza M, Riva E et al. Breakfast: a good habit, not a repetitive custom. J Int Med Res 2008; 36: 613–624.
Harma M, Ilmarinen J . Towards the 24-hour society—new approaches for aging shift workers? Scand J Work Environ Health 1999; 25: 610–615.
Knutson KL, Spiegel K, Penev P, Van Cauter E . The metabolic consequences of sleep deprivation. Sleep Med Rev 2007; 11: 163–178.
Knutsson A, Akerstedt T, Jonsson B, Orth-Gomer K . Increased risk of ischaemic heart disease in shift workers. Lancet 1986; 12: 89–92.
Stunkard AJ, Allison KC, Lundgren JD, O’Reardon JP . A biobehavioural model of the night eating syndrome. Obes Rev 2009; 10 (Suppl 2): 69–77.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW . Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009; 17: 2100–2102.
Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes (Lond) 2010; 34: 1589–1598.
Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S . Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA 2009; 106: 21453–21458.
Damiola F, Le MN, Preitner N, Kornmann B, Fleury-Olela F, Schibler U . Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000; 14: 2950–2961.
Satoh Y, Kawai H, Kudo N, Kawashima Y, Mitsumoto A . Time-restricted feeding entrains daily rhythms of energy metabolism in mice. Am J Physiol Regul Integr Comp Physiol 2006; 290: R1276–R1283.
Edery I . Circadian rhythms in a nutshell. Physiol Genomics 2000; 3: 59–74.
Takahashi JS, Hong HK, Ko CH, McDearmon EL . The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 2008; 9: 764–775.
Bass J, Takahashi JS . Circadian integration of metabolism and energetics. Science 2010; 330: 1349–1354.
Bray MS, Young ME . Regulation of fatty acid metabolism by cell autonomous circadian clocks: time to fatten up on information? J Biol Chem 2011; 286: 11883–11889.
Tsai JY, Kienesberger PC, Pulinilkunnil T . Sailors MH, Durgan DJ, Villegas-Montoya C, et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem 2010; 285: 2918–2929.
Young M, Razeghi P, Taegtmeyer H . Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res 2001; 88: 1142–1150.
Young M, Razeghi P, Cedars A, Guthrie P, Taegtmeyer H . Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res 2001; 89: 1199–1208.
Durgan D, Trexler N, Egbejimi O, McElfresh T, Suk H, Petterson L et al. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem 2006; 281: 24254–24269.
Durgan D, Smith J, Hotze M, Egbejimi O, Cuthbert K, Zaha V et al. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am J Physiol Heart Circ Physiol 2006; 290: H2480–H2497.
Young M, Patil S, Ying J, Depre C, Ahuja H, Shipley G et al. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J 2001; 15: 833–845.
Mistlberger RE . Neurobiology of food anticipatory circadian rhythms. Physiol Behav 2011; 104: 535–545.
Durgan DJ, Young ME . The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res 2010; 106: 647–658.
Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM . Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 2005; 55: 12–23.
Brown AM, Julian T . The body temperature response of two inbred strains of mice to handling, saline and amphetamine. Int J Neuropharmacol 1968; 7: 531–541.
Kvetnansky R, Sabban EL . Stress and molecular biology of neurotransmitter-related enzymes. Ann N Y Acad Sci 1998; 851: 342–356.
Kramer K, van de Weerd H, Mulder A, van Heijningen C, Baumans V, Remie R et al. Effect of conditioning on the increase of heart rate and body temperature provoked by handling in the mouse. ATLA: Altern Lab Anim 2004; 32: 177–181.
Poole T . Happy animals make good science. Lab Anim 1997; 31: 116–124.
Sujino M, Furukawa K, Koinuma S, Fujioka A, Nagano M, Iigo M et al. Differential Entrainment of Peripheral Clocks in the Rat by Glucocorticoid and Feeding. Endocrinology 2012; 153: 2277–2286.
Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U . Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 2001; 20: 7128–7136.
Hill JO . Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev 2006; 27: 750–761.
Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech 2011; 4: 733–745.
Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP . The continuing epidemics of obesity and diabetes in the United States. JAMA 2001; 286: 1195–1200.
Hussain MM, Pan X . Clock genes, intestinal transport and plasma lipid homeostasis. Trends Endocrinol Metab 2009; 20: 177–185.
Acknowledgements
This work was supported by the National Heart, Lung and Blood Institute (HL-074259 and HL-106199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Bray, M., Ratcliffe, W., Grenett, M. et al. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes 37, 843–852 (2013). https://doi.org/10.1038/ijo.2012.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.137
Keywords
This article is cited by
-
Maternal melatonin levels and temporal dietary intake: results from MY-CARE cohort study
BMC Pregnancy and Childbirth (2023)
-
Cardiomyocyte-specific disruption of the circadian BMAL1–REV-ERBα/β regulatory network impacts distinct miRNA species in the murine heart
Communications Biology (2023)
-
High-protein diet prevents fat mass increase after dieting by counteracting Lactobacillus-enhanced lipid absorption
Nature Metabolism (2022)
-
Induction of internal circadian desynchrony by misaligning zeitgebers
Scientific Reports (2022)
-
Association Between Sleep Pattern, Anthropometric Indicators, and Metabolic Risk Factors
Sleep and Vigilance (2022)