Abstract
Aims/hypothesis:
Visceral and intermuscular adipose tissue (IMAT) depots account for most obesity-related metabolic and cardiovascular complications. Muscle satellite cells (SCs) are mesenchymal stem cells giving rise to myotubes and also to adipocytes, suggesting their possible contribution to IMAT origin and expansion. We investigated the myogenic differentiation of SCs and the adipogenic potential of both preadipocytes and SCs from genetically obese Zucker rats (fa/fa), focusing on the role of Wnt signaling in these differentiation processes.
Methods:
SCs were isolated by single-fiber technique from flexor digitorum brevis muscle and preadipocytes were extracted from subcutaneous adipose tissue (AT). Morphological features and gene expression profile were evaluated during in vitro myogenesis and adipogenesis. Wingless-type MMTV integration site family member 10b (Wnt10b) expression was quantified by quantitative PCR in skeletal muscle and AT.
Results:
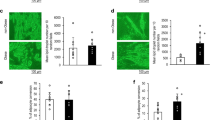
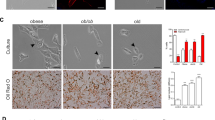
We did not observe any difference in the proliferation rate and in the myogenic differentiation of SCs from obese and lean rats. However, a decreased insulin-induced glucose uptake was present in myotubes originating from fa/fa rats. Under adipogenic conditions, preadipocytes and SCs of obese animals displayed an enhanced adipogenesis. Wnt10b expression was reduced in obese rats in both muscle and AT.
Conclusions/interpretation:
Our data suggest that the increase in different fat depots including IMAT and the reduced muscle insulin sensitivity, the major phenotypical alteration of obese Zucker rats, could be ascribed to an intrinsic defect, either genetically determined or acquired, still present in both muscle and fat precursors. The involvement of Wnt10b as a regulator of both adipogenesis and muscle-to-fat conversion is suggested.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003; 26: 372–379.
Goodpaster BH, Thaete FL, Kelley DE . Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000; 71: 885–892.
Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 2009; 297: E987–E998.
Charge SB, Rudnicki MA . Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004; 84: 209–238.
Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I . Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 2002; 37: 757–767.
Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005; 122: 289–301.
Kuang S, Rudnicki MA . The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 2008; 14: 82–91.
Asakura A, Komaki M, Rudnicki M . Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001; 68: 245–253.
De Coppi P, Milan G, Scarda A, Boldrin L, Centobene C, Piccoli M et al. Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia 2006; 49: 1962–1973.
Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z . Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci 2004; 117: 5393–5404.
Kuang S, Kuroda K, Le Grand F, Rudnicki MA . Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007; 129: 999–1010.
Zammit PS, Partridge TA, Yablonka-Reuveni Z . The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 2006; 54: 1177–1191.
Kausch C, Krutzfeldt J, Witke A, Rettig A, Bachmann O, Rett K et al. Effects of troglitazone on cellular differentiation, insulin signaling, and glucose metabolism in cultured human skeletal muscle cells. Biochem Biophys Res Commun 2001; 280: 664–674.
Sanna M, Franzin C, Pozzobon M, Favaretto F, Rossi CA, Calcagno A et al. Adipogenic potential of skeletal muscle satellite cells. Clinical Lipidology 2009; 4: 245–265.
Yada E, Yamanouchi K, Nishihara M . Adipogenic potential of satellite cells from distinct skeletal muscle origins in the rat. J Vet Med Sci 2006; 68: 479–486.
Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA . Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev 2002; 123: 649–661.
Bray GA . The Zucker-fatty rat: a review. Fed Proc 1977; 36: 148–153.
Guerre-Millo M . Regulation of ob gene and overexpression in obesity. Biomed Pharmacother 1997; 51: 318–323.
Durschlag RP, Layman DK . Skeletal muscle growth in lean and obese Zucker rats. Growth 1983; 47: 282–291.
Shapira JF, Kircher I, Martin RJ . Indices of skeletal muscle growth in lean and obese Zucker rats. J Nutr 1980; 110: 1313–1318.
Wardlaw GM, Kaplan ML, Lanza-Jacoby S . Effect of treadmill training on muscle oxidative capacity and accretion in young male obese and nonobese Zucker rats. J Nutr 1986; 116: 1841–1852.
Peterson JM, Bryner RW, Alway SE . Satellite cell proliferation is reduced in muscles of obese Zucker rats but restored with loading. Am J Physiol Cell Physiol 2008; 295: C521–C528.
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A . Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009; 20: 16–24.
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL et al. Inhibition of adipogenesis by Wnt signaling. Science 2000; 289: 950–953.
Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee Jr RE et al. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell 2005; 16: 2039–2048.
Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA . Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 1995; 31: 773–779.
Milan G, Dalla Nora E, Pilon C, Pagano C, Granzotto M, Manco M et al. Changes in muscle myostatin expression in obese subjects after weight loss. J Clin Endocrinol Metab 2004; 89: 2724–2727.
Argiles JM, Busquets S, Alvarez B, Lopez-Soriano FJ . Mechanism for the increased skeletal muscle protein degradation in the obese Zucker rat. J Nutr Biochem 1999; 10: 244–248.
Crettaz M, Prentki M, Zaninetti D, Jeanrenaud B . Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem J 1980; 186: 525–534.
Czech MP, Richardson DK, Becker SG, Walters CG, Gitomer W, Heinrich J . Insulin response in skeletal muscle and fat cells of the genetically obese Zucker rat. Metabolism 1978; 27: 1967–1981.
Penicaud L, Ferre P, Terretaz J, Kinebanyan MF, Leturque A, Dore E et al. Development of obesity in Zucker rats. Early insulin resistance in muscles but normal sensitivity in white adipose tissue. Diabetes 1987; 36: 626–631.
King PA, Horton ED, Hirshman MF, Horton ES . Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J Clin Invest 1992; 90: 1568–1575.
Zarjevski N, Doyle P, Jeanrenaud B . Muscle insulin resistance may not be a primary etiological factor in the genetically obese fa/fa rat. Endocrinology 1992; 130: 1564–1570.
Rossi CA, Pozzobon M, Ditadi A, Archacka K, Gastaldello A, Sanna M et al. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PlosOne 2010; 5: e8523.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967.
Gregoire FM, Johnson PR, Greenwood MR . Comparison of the adipoconversion of preadipocytes derived from lean and obese Zucker rats in serum-free cultures. Int J Obes Relat Metab Disord 1995; 19: 664–670.
Bourgeois F, Goldstein AL, Johnson PR . Lipogenesis in primary cultures of adipoblasts derived from genetically obese Zucker rats. Metabolism 1983; 32: 673–680.
Bai Y, Zhang S, Kim KS, Lee JK, Kim KH . Obese gene expression alters the ability of 30A5 preadipocytes to respond to lipogenic hormones. J Biol Chem 1996; 271: 13939–13942.
Rhee SD, Sung YY, Jung WH, Cheon HG . Leptin inhibits rosiglitazone-induced adipogenesis in murine primary adipocytes. Mol Cell Endocrinol 2008; 294: 61–69.
Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002; 277: 30998–31004.
Akimoto T, Ushida T, Miyaki S, Akaogi H, Tsuchiya K, Yan Z et al. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem Biophys Res Commun 2005; 329: 381–385.
Acknowledgements
The excellent technical assistance of Sonia Leandri and Cinzia Centobene is greatly appreciated. This work was supported by grant RF-ASR-2007-628447 from the Italian Ministry of Health given to RV, by grant 2007BRR57M_004 from the Italian Ministry of University to GF, by grant RF-VEN-2005-132819 given to RV and by a grant from Città della Speranza.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Scarda, A., Franzin, C., Milan, G. et al. Increased adipogenic conversion of muscle satellite cells in obese Zucker rats. Int J Obes 34, 1319–1327 (2010). https://doi.org/10.1038/ijo.2010.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.47
Keywords
This article is cited by
-
Adipogenic progenitors in different organs: Pathophysiological implications
Reviews in Endocrine and Metabolic Disorders (2022)
-
Dysmetabolic adipose tissue in obesity: morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects
Journal of Endocrinological Investigation (2021)
-
The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function
Stem Cell Research & Therapy (2019)
-
Characterization and utilization of the flexor digitorum brevis for assessing skeletal muscle function
Skeletal Muscle (2018)
-
A multiscale modeling framework for studying the mechanobiology of sarcopenic obesity
Biomechanics and Modeling in Mechanobiology (2017)