Abstract
Activation of the intrarenal renin–angiotensin system (RAS) has a critical role in the pathophysiology of the circadian rhythm of blood pressure (BP) and renal injury, independent of circulating RAS. Although it is clear that the circulating RAS has a circadian rhythm, reports of a circadian rhythm in tissue-specific RAS are limited. Clinical studies evaluating intrarenal RAS activity by urinary angiotensinogen (AGT) levels have indicated that urinary AGT levels were equally low during both the daytime and nighttime in individuals without chronic kidney disease (CKD) and that urinary AGT levels were higher during the daytime than at nighttime in patients with CKD. Moreover, urinary AGT levels of the night-to-day (N/D) ratio of urinary AGT were positively correlated with the levels of N/D of urinary protein, albumin excretion and BP. In addition, animal studies have demonstrated that the expression of intrarenal RAS components, such as AGT, angiotensin II (AngII) and AngII type 1 receptor proteins, increased and peaked at the same time as BP and urinary protein excretion during the resting phase, and the amplitude of the oscillations of these proteins was augmented in a chronic progressive nephritis animal compared with a control. Thus, the circadian rhythm of intrarenal RAS activation may lead to renal damage and hypertension, which both are associated with diurnal variations in BP. It is possible that augmented glomerular permeability increases AGT excretion levels into the tubular lumen and that circadian fluctuation of glomerular permeability influences the circadian rhythm of the intrarenal RAS.
Similar content being viewed by others
Introduction
Disturbance of circadian rhythm is associated with an increased risk for developing various disorders, including sleep disorders, metabolic syndrome and cancer; and estimation methods for the human circadian phase have been developed.1
Blood pressure (BP) is one of the clinical conditions that develop circadian rhythm. Hypertension and disturbance of the circadian rhythm of BP contribute to cardiovascular events and renal damage,2, 3, 4, 5 and some clinical studies suggest that bedtime chronotherapy decreases BP and reduces cardiovascular events.6 Tubular sodium reabsorption, which is stimulated by activation of the intrarenal renin–angiotensin system (RAS), contributes to the genesis of disturbed circadian BP rhythm.7 It is well known that the circulating RAS has a circadian rhythm.8, 9, 10 Alternatively, whether the circadian rhythm of the intrarenal RAS actually exists and how the circadian rhythm of the intrarenal RAS may associate with renal damage and the circadian rhythm of BP is not clear. The purpose of this review was to clarify the above issues.
Contribution of hypertension and disturbances in the circadian rhythm of BP in cardiovascular disease and renal damage
Hypertension is one of the major risk factors for cardiovascular disease (CVD), stroke and end-stage renal disease.2, 11 Circadian BP rhythms are classified as having extreme dipper, dipper, non-dipper or riser patterns when the night-to-day (N/D) ratio of systolic BP corresponds to <0.80, 0.80 to <0.90, 0.90–1 and >1, respectively.12 Growing evidence has recently demonstrated that disruption of diurnal BP variations is an additional risk factor for CVD, stroke and end-stage renal disease, independent of BP elevation.3, 4, 5 In fact, Ohkubo et al.3 indicated that each 5% decrease in the decline in nocturnal systolic/diastolic BP was associated with an approximately 20% greater risk of cardiovascular mortality in 1542 individuals from the general Japanese population. Kario et al.4 reported that intracranial hemorrhage was more common in riser patterns (29% of stroke) than in other subgroups (7.7% of strokes, P=0.04), and ischemic strokes that occurred during sleep occurred more frequently in the extreme dipper group (27%) than in the other groups (8.6%, P=0.11) in a study evaluating 575 older Japanese patients with sustained hypertension. In addition, Agarwal and Andersen5 demonstrated that when the N/D ratio of systolic ambulatory BP was divided into the tertiles (N/D ratio <0.93, 0.93–0.99 and >0.99) in a cohort study of 217 veterans with chronic kidney disease (CKD), the patients in the lowest tertiles had the least incidences of end-stage renal disease events compared with those in the higher tertiles (P=0.016).
Factors associated with disturbances in the circadian rhythm of BP
Some factors are associated with disturbed circadian rhythm of BP. Namely, disruptions in sleeping rhythms caused by sleep apnea syndrome, stroke or working a day-night shift cause disturbances in the circadian rhythm of BP. In addition, disturbances in the secretion rhythm of vasoactive hormones, including pheochromocytoma and Cushing syndrome are also associated with a disturbed circadian rhythm of BP. However, an impaired renal capacity for sodium excretion, or so-called salt-sensitive hypertension, is the major pathophysiological mechanism causing a disturbed circadian rhythm of BP13 (Table 1). The mechanisms involved in salt-sensitive hypertension include reduced ultrafiltration capacity or enhanced tubular sodium reabsorption. Farmer et al.14 estimated the ambulatory BP in 380 patients with essential hypertension and showed that the prevalence of a non-dipping pattern in diurnal BP rhythm increased as plasma creatinine level increased, irrespective of the number of antihypertensives taken by patients. Goto et al.15 indicated that positive relationships existed between changes in creatinine clearance and an increase in the N/D ratio of the mean BP before and after nephrectomy in healthy subjects who underwent unilateral nephrectomy for kidney donation. These results support the observation that insufficient natriuresis due to a decrease in the glomerular filtration elevates nocturnal BP. In addition, Fukuda et al.7 investigated the contribution of renal proximal tubular angiotensinogen (AGT) production to the enhancement of fractional tubular sodium reabsorption and the non-dipper circadian BP rhythm in 40 patients with primary immunoglobulin A (IgA) nephropathy and showed that immunostaining for proximal tubular AGT was significantly and positively correlated with fractional tubular sodium reabsorption and the N/D ratio of BP. These results suggest that tubular sodium reabsorption is stimulated by the intrarenal RAS and contributes to the genesis of the non-dipper BP rhythm (Figure 1).7
Relationships of the proximal tubular angiotensinogen (AGT) expression level with tubular sodium reabsorption to filtered sodium load ratio (FRNa) (a) and the night/day ratio of the mean arterial pressure (MAP) (b) in 40 patients with immunoglobulin A (IgA) nephropathy. Reprinted from Fukuda et al.7 with permission of the publisher. Copyright 2012, Wolters Kluwer Health.
The independent roles of the circulating RAS and tissue-specific RAS
It has been well known for many years that the circulating RAS has a critical role in the regulation of arterial pressure and sodium homeostasis.10, 16 In recent years, the focus of interest on the role of the RAS in the pathophysiology of hypertension and organ injury has changed to the local RAS in specific tissues. In the kidney, all RAS components are present, and it has been clarified that activation of the intrarenal RAS has a critical role in the pathophysiology of CKD and hypertension, independent of the circulating RAS.16, 17
The role and circadian rhythm of the circulating RAS and factors affecting the circulating RAS
Because renin was first identified by Tigerstedt and Bergnmann in 1898, the RAS has been extensively studied. Renin, a key molecule in the systemic RAS, is secreted as outlined below, and it has been clarified that the circulating RAS has crucial roles in regulating body fluid volume and maintaining BP. Renin is secreted from the juxtaglomerular apparatus as follows: (1) by stretch stimulation from baroreceptors (or stretch receptors) in the wall of the afferent arteriole,18 (2) by pressure stimulation from cardiac and arterial baroreceptors, which regulate sympathetic neural activity and circulating catecholamine levels19, 20 and (3) by the chloride concentration in the fluid delivered to the macula densa.21, 22
Since the 1960s, it has been well known that the circulating RAS has a circadian rhythm. Gordon et al.9 investigated six normal subjects who were continuously recumbent and who received identical feedings. They clarified that plasma renin activity in the morning is higher than that in the afternoon and that there is a normal diurnal rhythm in plasma renin activity that is unexplained by diurnal changes in diet or posture (Figure 2a).9 Moreover, Kala et al.10 examined the circulating angiotensin II (AngII) concentration from 10 healthy men aged 22–29 and 10 healthy women aged 19–23 and indicated that plasma AngII exhibits a diurnal variation, with the highest and lowest values detected at 0800 and 2000 hours, respectively (Figure 2b).
Circadian rhythm of circulating renin–angiotensin system. (a) Plasma renin activity (PRA): serial PRA values were obtained from six normal subjects who were continuously recumbent and received identical feedings at 4 h intervals. Reprinted from Gordon et al.9 with permission of the publisher. Copyright 1966, American Society for Clinical Investigation. (b) Plasma angiotensin II (AngII): serial AngII values were obtained at 4-h intervals from 20 normal subjects (10 men and 10 women) who received normal meals at 0830, 1100 and 1700 hours. Reprinted from Kala et al.10 with permission of the publisher. Copyright 1973, Taylor Francis.
The role and circadian rhythm of tissue-specific RAS and factors affecting tissue-specific RAS
Although it is clear that the circulating RAS has a circadian rhythm, reports of a circadian rhythm in tissue-specific RAS are limited.
The role and circadian rhythm of cardiac RAS components and factors affecting cardiac RAS components
The cardiac RAS has several important roles, including inotropic effects, hypertrophic effects, mechanical stretch, remodeling and apoptosis, independent of the circulating RAS. The predominant physiological role of the cardiac RAS appears to be the maintenance of an appropriate cellular milieu balancing stimuli, inducing and inhibiting cell growth and proliferation as well as mediating adaptive responses to myocardial stress.
Naito et al.23 investigated the circadian rhythm of cardiac RAS components in spontaneously hypertensive rats (SHR) compared with normotensive control Wistar–Kyoto (WKY) rats. They indicated that the cardiac messenger RNA (mRNA) of RAS components showed a circadian rhythm in both SHR and WKY rats. In addition, they demonstrated that the amplitudes of these circadian fluctuations were greater in the SHR than in the WKY rats and that the mRNA levels of the RAS components increased in the SHR compared with those in WKY rats at many time points (Figure 3). In this study, although the changes in cardiac mRNA expression of renin and AGT following the administration of the AngII type 1 receptor (AT1R) antagonist did not reach statistical significance, treatment upregulated AT1a receptor mRNA expression and downregulated angiotensin converting enzyme (ACE) mRNA expression at specific time points in the SHR group alone (Figure 4).23
Relative expression levels of cardiac renin–angiotensin system in Wistar–Kyoto (WKY) rats and spontaneously hypertensive rats (SHR). Expression of (a) renin, (b) angiotensinogen (ATNG), (c) angiotensin converting enzyme (ACE) and (d) angiotensin II type 1a receptor (AT1aR). All expression values were normalized against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Trough values for the WKY group were arbitrarily set to 1. Data are represented as the mean±SEM values in SHR (●) and WKY rats (○). *P<0.05 vs. WKY rats. Reprinted from Naito et al.23 with permission of the publisher. Copyright 2002, American Heart Association.
Relative expression levels of cardiac renin–angiotensin system in control spontaneously hypertensive rats (SHR) and candesartan (angiotensin II receptor blocker)-treated SHR. Expression of (a) renin, (b) angiotensinogen (ATNG), (c) angiotensin converting enzyme (ACE) and (d) angiotensin II type 1a receptor (AT1aR). All expression values were normalized against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Trough values for the control SHR group were arbitrarily set to 1. Data are represented as the mean±SEM values in control SHR (●) and candesartan treated SHR (▾). *P<0.05 vs. control SHR. Reprinted from Naito et al.23 with permission of the publisher. Copyright 2002, American Heart Association.
The role and circadian rhythm of intrarenal RAS components and factors affecting intrarenal RAS components
The role of intrarenal RAS components
It has been clarified that intrarenal RAS activation has an important role in the development of renal damage.24, 25, 26, 27, 28, 29 In addition, the contribution of the intrarenal RAS to salt-sensitive hypertension and nocturnal hypertension has been shown. Konishi et al.30 indicated that when the salt-sensitivity index was calculated as the reciprocal of the slope of the pressure-natriuresis line, drawn by linking 2 data points obtained during consumption of an ordinary salt diet (12 g per day) and a low-salt diet (5 g per day) in IgA nephropathy patients, the sodium sensitivity index positively correlated with the changes in the logarithmic urinary AGT/creatinine ratio as well as glomerulosclerosis. These data suggest that the inappropriate augmentation of intrarenal AGT induced by salt and the associated renal damage contribute to the development of salt-sensitive hypertension in patients with IgA nephropathy. In addition, Fukuda et al.7 demonstrated that immunostaining for proximal tubular AGT increased in IgA nephropathy patients compared with that in control individuals and directly correlated with the fractional tubular sodium reabsorption and the N/D ratio of BP. These data concluded that tubular sodium reabsorption is stimulated by intrarenal RAS activation and contributes to the genesis of the non-dipper BP rhythm.7
Factors affecting intrarenal RAS components
Several investigators have demonstrated that intrarenal reactive oxygen species (ROS) are associated with intrarenal RAS activation and have a pivotal role in the development of renal damage such as IgA nephropathy and diabetes mellitus (DM) in human subjects and animal models.31, 32, 33, 34 For example, Miyata et al.33 reported that ZDF obese rats, an animal model of type 2 DM, developed DM at 12 weeks, an increase in urinary 8-isoprostane (a marker of oxidative stress) levels at 15 weeks, and an elevation of AGT in the kidney at 18 weeks and they proposed the sequential activation of the ROS–RAS axis in DM.33 Moreover, a subsequent in vitro experiment showed the precise interaction between ROS and RAS in hyperglycemic conditions such as DM.35
Zalucky et al.36 indicated decreased effective renal plasma flow response to AngII challenge (a marker of renal RAS activity) in obstructive sleep apnea patients compared with that in control subjects. They concluded that the severity of nocturnal hypoxemia influenced the magnitude of renal RAS activation independently of obesity in obstructive sleep apnea patients. Moreover, Nicholl et al.37 reported that when obstructive sleep apnea was ameliorated by continuous positive airway pressure, the renal RAS activity was downregulated.
Moreover, cardiac disease is also an important factor affecting the activation of the intrarenal RAS. Wen et al.38 estimated the mechanism of renal impairment after myocardial infarction and clarified that renal impairment was associated with intrarenal RAS activation, using measures such as increased intrarenal AngII levels and enhanced expressions of AGT mRNA and AngII receptor mRNA and protein.
Circadian rhythm of intrarenal RAS components
Clinical studies
Urinary AGT has been used in many animal models and clinical studies.24, 25, 26, 27, 28, 29 For example, we measured urinary AGT concentration in 70 hypertensive patients (39 treated with RAS blockers and 31 not treated with RAS blockers) and 36 normotensive subjects and clarified that urinary AGT/urinary creatinine concentration was significantly greater in hypertensive patients not treated with RAS blockers than in normotensive subjects. Additionally, we showed that patients treated with RAS blockers exhibited a marked attenuation of this augmentation.28 In addition, Fuwa et al.39 demonstrated that a lower sodium balance was produced by add-on hydrochlorothiazide to AngII receptor blocker (ARB) treatment with reduction of urinary AGT excretion (intrarenal RAS suppression), leading to the resolution of nocturnal hypertension. These results indicate that urinary AGT has been confirmed as an accurate biomarker that reflects intrarenal RAS activity.
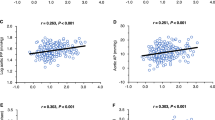
However, little has been reported regarding the existence of circadian rhythm of the intrarenal RAS until recently. Nishijima et al.40 investigated the circadian rhythm of the intrarenal RAS by using urinary AGT and reported that urinary AGT levels were unchanged at three time points (0900, 1300 and 1600 hours) in 24 healthy volunteers and at three time points (0600, 1200 and 1600 hours) in 24 healthy volunteers and eight patients with CKD (Figure 5).41 However, these studies did not analyze nocturnal intrarenal RAS activity, and the number of patients with CKD was limited. Therefore, we investigated the circadian rhythm of urinary AGT that was divided and collected in the daytime (0600 to 2100 hours) and nighttime (2100 to 0600 hours) in 14 individuals without CKD and in 36 patients with CKD. The circadian rhythm patterns of BP in patients with CKD were as follows: dippers in 6, non-dippers in 20 and risers in 10, and no extreme dippers were found. We observed that urinary AGT levels were equally low during both the daytime and nighttime in individuals without CKD. In addition, we showed that urinary AGT levels were significantly higher in patients with CKD than in those without CKD in both the daytime and nighttime and that urinary AGT levels were higher during the daytime than at nighttime in patients with CKD. Moreover, we found that urinary AGT levels during the daytime and nighttime were elevated to similar levels in patients with CKD with the ‘riser’ BP pattern and that the levels of N/D ratio of urinary AGT were positively correlated with the levels of N/D of urinary protein and albumin excretion and BP. Thus, we concluded that the circadian rhythm of intrarenal RAS activation may lead to renal damage and hypertension, both of which are associated with diurnal variations in BP, and that it is possible that urinary AGT-guided antihypertensive therapy improves renal prognosis42 (Figure 6; Table 2). It is difficult to evaluate the contribution of progression of renal damage with the extreme dipper BP pattern because this pattern is less frequent (11.1% in Cha et al.’s report43 and 0% in our report42). In addition, Kario et al.4 reported that it is possible that extreme dipping of nocturnal BP is related to silent cerebral ischemia through hypoperfusion during sleep or an exaggerated morning rise of BP in older Japanese hypertensive patients, and Bastos et al.44 revealed that patients with cardiovascular events (acute coronary syndromes, strokes, acute heart failure and arrhythmias) more frequently showed extreme dipper patterns than patients without cardiovascular events in resistant hypertensive patients. On the other hand, the relationship between the progression of renal damage and the extreme dipper pattern has not been clarified until now. We are looking forward to larger clinical trials in the near future, which we hope will further clarify this relationship.
Circadian rhythm of the intrarenal renin–angiotensin system based on urinary angiotensinogen (AGT) excretion in healthy volunteers (a) and in patients with chronic kidney disease (CKD) (b). Urinary AGT excretion of 24 healthy volunteers and 8 patients with CKD did not show a circadian rhythm at three time points (0600, 1200 and 1600 hours). Reprinted from Nishijima et al.41 with permission of the publisher. Copyright 2012, SAGE Publication.
Changes in urinary angiotensinogen to creatinine ratio (U-AGT/Cr) comparing daytime and nighttime values in (a) all patients with chronic kidney disease (CKD), (b) CKD non-riser patients and (c) riser patients with CKD. The solid line indicates the change in U-AGT/Cr levels in non-riser patients with CKD, and the dotted line indicates the change in U-AGT/Cr levels in riser patients with CKD. In patients with CKD, U-AGT/Cr levels during the daytime were significantly higher than those during the nighttime. In non-riser patients with CKD, the change in U-AGT/Cr levels followed the same pattern as that of patients with CKD. Conversely, U-AGT/Cr levels during the nighttime did not decrease compared with those during the daytime, and the circadian rhythm of U-AGT/Cr disappeared in riser patients with CKD. Reprinted from Isobe et al.42 with permission of the publisher. Copyright 2015, Japanese Society of Nephrology.
Animal studies
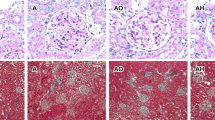
The circadian rhythm of other intrarenal RAS components in addition to AGT and a detailed evaluation of circadian rhythms of the intrarenal RAS have not been described. Furthermore, it is not clear whether the administration of AT1R blockers influences the circadian rhythm of intrarenal RAS components. Therefore, an animal study was conducted using rats with chronic progressive nephritis to address these concerns.45 Chronic progressive anti-thymocyte serum (ATS) nephritis was induced by two consecutive injections of ATS 1 week apart after uninephrectomy (group A), and a comparison was performed with control rats (group C). Treatment intervention was performed by administering olmesartan medoxomil (ARB) (group AO) or hydralazine (a vasodilator) (group AH) as described previously.46 The levels of intrarenal RAS components were evaluated every 6 h.
The expression levels of intrarenal AGT, AngII and AT1R increased in group A and peaked at the same time as BP and urinary protein excretion during the resting phase. The amplitude of circadian fluctuation of these proteins (peak to trough (P/T) ratio) was higher in group A than in group C. Both BP levels and circadian fluctuation of these proteins decreased in the AO and AH groups. However, renal function, proteinuria and augmentation of intrarenal RAS components were reduced in the AO group alone (Figure 7). In contrast, protein expression levels of intrarenal renin and prorenin showed the same tendency as that of AGT, AngII and AT1R, although fluctuation in these levels was augmented in group AO (Figure 8). Protein expression levels and fluctuation of ACE and (pro)renin receptor [(P)RR] did not differ among the groups (Figure 9).
Circadian rhythm of angiotensinogen (AGT), angiotensin II (AngII) and AngII type 1 receptor (AT1R) in the kidney. (a) Representative immunoblot of AGT protein expression. (b) Densitometric ratios of AGT/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) bands are shown. The amplitude of the fluctuation of AGT expression in group A is greater than that in group C and that of the AO and AH groups is diminished (Peak to trough [P/T] ratio of AGT expression in C, 1.13; A, 1.47; AO, 1.24; AH, 1.22). (c) Representative immunostaining for AGT expression at Zeitgeber time (ZT) 6. Immunostaining for AGT in group C reveals slight expression in proximal tubular cells and dramatically increased expression in group A. Although intrarenal AGT expression is reduced in group AO, the expression of AGT is not significantly decreased in group AH compared with that in group A. Original magnification × 400. (d) Representative immunostaining for AngII protein expression at ZT 6. Immunostaining for AngII in group C is weak and is observed mainly in some distal tubular cells (arrowheads). Immunostaining for AngII in group A is significantly enhanced in the proximal (arrows) and distal tubules (arrowheads), and AngII in group AO (but not in group AH) is decreased compared with that in group A. Original magnification × 400. (e) The degree of AngII immunoreactivity is shown. The average immunoreactivity of AngII in 20 microscopic fields for each slide is quantitatively evaluated by a semi-automatic image analysis system using Image Pro Plus software. The amplitude of the fluctuation of AngII expression in group A is greater than that in group C and peaked at ZT 6. In addition, the oscillations in the AO and AH groups diminished (P/T ratio of AngII expression in C, 1.03; A, 1.26; AO, 1.14; AH, 1.17). (f) Representative immunoblot of AT1R protein expression. (g) Densitometric ratios of AT1R/GAPDH bands are shown. The amplitude of fluctuations of AT1R expression in group A is greater than that in group C, whereas that in the AO and AH groups is diminished (P/T ratio of AT1R protein expression in C, 1.36; A, 1.51; AO, 1.37; AH, 1.26). The solid line with open circles indicates group C, the solid line with closed circles indicates group A, the dotted line with closed squares indicates group AO, and the dashed line with open triangles indicates group AH. Data are represented as means±s.e. #P<0.05 group C vs. group A. *P<0.05 group A vs. group AO. C, group C; A, group A; AO, group AO; AH, group AH. Reprinted from Isobe et al.45 with permission of the publisher. Copyright 2016, Nature Publishing Group. A full color version of this figure is available at Hypertension Research online.
Circadian rhythm of renin and prorenin levels in the kidney. (a) Representative immunoblot of renin and prorenin protein expression. (b) Densitometric ratios of renin and prorenin/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) bands are shown. The amplitude of fluctuations of renin and prorenin expression in group AO is greater than that in the other groups (peak to trough [P/T] ratio of renin in C, 1.30; A, 1.34; AO, 1.82; AH, 1.47; and P/T ratio of prorenin in C, 1.47; A, 1.40; AO, 1.59; AH, 1.39). The solid lines with open circles indicate group C, the solid lines with closed circles indicate group A, the dotted lines with closed squares indicate group AO, and the dashed lines with open triangles indicate group AH. Data represent means±s.e. #P<0.05 group C vs. group A. *P<0.05 group A vs. group AO. (c) Representative immunostaining for total renin protein at Zeitgeber time (ZT) 6. In group C, significant immunostaining for total renin is observed in the juxtaglomerular cells (arrows) and is faintly observed in some collecting ducts and connecting tubules (arrowheads). In contrast, immunostaining for total renin is observed only in the collecting ducts and connecting tubules in groups A and AH. Immunostaining of total renin in juxtaglomerular cells in group AO is observed as clearly as that in group C. However, the immunostaining intensity in the collecting ducts are markedly decreased compared with those in groups A and AH. Original magnification × 400. C, group C; A, group A; AO, group AO; AH, group AH. Reprinted from Isobe et al.45 with permission of the publisher. Copyright 2016, Nature Publishing Group. A full color version of this figure is available at Hypertension Research online.
Circadian rhythm of (pro)renin receptor [(P)RR] and angiotensin converting enzyme (ACE) in the kidney. (a) Representative immunoblot of (P)RR protein expression. (b) Densitometric ratios of (P)RR/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) bands are shown. The degree of oscillation is similar among the groups (peak to trough [P/T] ratio of (P)RR protein expression in C, 1.37; A, 1.24; AO, 1.28; AH, 1.23). (C) Representative immunostaining for (P)RR at Zeitgeber time (ZT) 6. The collecting duct and connecting tubular cells were observed, and the levels of immunostaining were not different among the groups. Original magnification × 400. (d) Representative immunoblot of ACE protein expression. (e) Densitometric ratios of ACE/GAPDH bands are shown. The degree of oscillation was similar among the groups (P/T ratio of ACE expression in C, 1.16; A, 1.19; AO, 1.28; AH, 1.21). (f) Representative immunostaining for ACE protein at ZT 6. ACE was immunostained at ZT 6 in the brush border of proximal tubules (arrows) and in the endothelium of the blood vessels (arrowheads). Immunostaining intensity for ACE in both lesions did not differ among the groups. Original magnification × 400. The solid line with open circles indicates group C, the solid line with closed circles indicates group A, the dotted line with closed squares indicates group AO, and the dashed line with open triangles indicates group AH. Data are represented as means±s.e. C, group C; A, group A; AO, group AO; AH, group AH. Reprinted from Isobe et al.45 with permission of the publisher. Copyright 2016, Nature Publishing Group. A full color version of this figure is available at Hypertension Research online.
These results indicate that renal damage may be linked to the activation of intrarenal RAS components, such as AGT, AngII and AT1R, independent of the amplitude of its oscillations and BP.
Possible pathogenetic mechanism of disturbances in the circadian rhythm of the intrarenal RAS
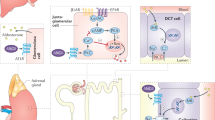
It is known that AGT in the kidney is produced in the proximal tubules.16 However, Matsusaka et al.47 demonstrated that AGT produced in the liver and filtered through the glomerular basement membrane is the primary source of intrarenal AngII. Some studies have reported that there is no evidence of a circadian rhythm of liver AGT and that no significant variations indicative of a circadian rhythm are found in plasma AGT.41, 45 Glomerular permeability is augmented by glomerular injury.48, 49 In addition, glomerular hypertension influences glomerular permeability more than systemic hypertension. Griffin et al.50 demonstrated the differences between intraglomerular BPs in 5/6 nephrectomized rats treated with an ACE inhibitor and a calcium channel blocker and that glomerular damage was ameliorated by the ACE inhibitor but not the calcium channel blocker because of the prevention of greater pressure transmission to the glomeruli. Moreover, glomerular hypertension increases AGT excretion levels in the tubular lumen.51 Therefore, it is possible that circadian fluctuation of glomerular permeability due to glomerular hypertension may influence the circadian rhythm of intrarenal AGT. Once the AGT is excreted into the tubular lumen, AngII is generated because all the components necessary for AngII synthesis are located in the tubules.16, 52, 53 This may explain why circadian fluctuations of the intrarenal RAS exist, why BP elevation, urinary protein excretion and intrarenal RAS activation occur in parallel in patients with CKD and in the chronic progressive nephritis model, and ultimately, why fluctuations in the intrarenal RAS are not related to those in the circulating RAS.
Conclusion
This review indicates that the circadian rhythm of the intrarenal RAS clearly exists. Moreover, intrarenal RAS activity in patients with CKD, as evaluated by urinary AGT levels, indicates that disturbances in the circadian rhythm of intrarenal RAS activation lead to renal damage, hypertension and variations in diurnal BP. Animal studies demonstrate that levels of the intrarenal RAS components such as AGT, AngII and AT1R proteins are increased and that the amplitude of oscillations of these proteins is augmented.
In conclusion, it is plausible that glomerular permeability and hypertension increases the levels of AGT excretion into the tubular lumen and that circadian fluctuation of glomerular permeability influences intrarenal RAS activation.
References
Matsumura R, Node K, Akashi M . Estimation methods for human circadian phase by use of peripheral tissues. Hypertens Res 2016; 39: 623–627.
Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J . Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36: 646–661.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y . Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189.
Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K . Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38: 852–857.
Agarwal R, Andersen MJ . Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69: 1175–1180.
Hermida RC, Ayala DE, Smolensky MH, Fernández JR, Mojón A, Portaluppi F . Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res 2016; 39: 277–292.
Fukuda M, Urushihara M, Wakamatsu T, Oikawa T, Kobori H . Proximal tubular angiotensinogen in renal biopsy suggests nondipper BP rhythm accompanied by enhanced tubular sodium reabsorption. J Hypertens 2012; 30: 1453–1459.
Hilfenhaus M . Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Arch Toxicol 1976; 17: 305–316.
Gordon RD, Wolfe LK, Island DP, Liddle GW . A diurnal rhythm in plasma renin activity in man. J Clin Invest 1966; 45: 1587–1592.
Kala R, Fyhrquist F, Eisalo A . Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest 1973; 31: 363–365.
Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C . Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005; 165: 923–928.
Kario K, Shimada K . Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens 2004; 26: 177–189.
Kimura G, Dohi Y, Fukuda M . Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res 2010; 33: 515–520.
Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P . An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant 1997; 12: 2301–2307.
Goto N, Uchida K, Morozumi K, Ueki T, Matsuoka S, Katayama A, Haba T, Tominaga Y, Fukuda M, Nakao A, Kimura G . Circadian blood pressure rhythm is disturbed by nephrectomy. Hypertens Res 2005; 28: 301–306.
Kobori H, Nangaku M, Navar LG, Nishiyama A . The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287.
Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H . Regulation of intrarenal angiotensin II in hypertension. Hypertension 2002; 39: 316–322.
Bock HA, Hermle M, Brunner FP, Thiel G . Pressure dependent modulation of renin release in isolated perfused glomeruli. Kidney Int 1992; 41: 275–280.
Freeman RH, Davis JO, Villarreal D . Role of renal prostaglandins in the control of renin release. Circ Res 1984; 54: 1–9.
Kopp U, DiBona GF . Interaction of renal beta 1-adrenoceptors and prostaglandins in reflex renin release. Am J Physiol 1983; 244: F418–F424.
Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP . Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol 1991; 260: F486–F493.
Kotchen TA, Luke RG, Ott CE, Galla JH, Whitescarver S . Effect of chloride on renin and blood pressure responses to sodium chloride. Ann Intern Med 1983; 98: 817–822.
Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Iwasaki T . Augmented diurnal variations of the cardiac renin-angiotensin system in hypertensive rats. Hypertension 2002; 40: 827–833.
Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, Togawa A, Suzuki S, Fujigaki Y, Nakagawa T, Nakamura Y, Suzuki F, Kitagawa M, Hishida A . Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Renal Physiol 2008; 295: F1512–F1518.
Kamiyama M, Zsombok A, Kobori H . Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. J Pharmacol Sci 2012; 119: 314–323.
Urushihara M, Ohashi N, Miyata K, Satou R, Acres OW, Kobori H . Addition of angiotensin II type 1 receptor blocker to CCR2 antagonist markedly attenuates crescentic glomerulonephritis. Hypertension 2011; 57: 586–593.
Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A . Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 2007; 18: 1558–1565.
Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG . Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 2009; 53: 344–350.
Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M . Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant 2011; 26: 170–177.
Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, Kishida M, Hitomi H, Kiyomoto H, Miyashita T, Mori N, Urushihara M, Kobori H, Imanishi M . Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension 2011; 58: 205–211.
Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T . Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun 2007; 22: 156–163.
Ohashi N, Urushihara M, Kobori H . Activated intrarenal reactive oxygen species and renin angiotensin system in IgA nephropathy. Minerva Urol Nefrol 2009; 61: 55–66.
Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H . Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol 2008; 35: 922–927.
Kamiyama M, Urushihara M, Morikawa T, Konishi Y, Imanishi M, Nishiyama A, Kobori H . Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int J Mol Sci 2013; 21:23045–23062.
Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS . High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 2002; 143: 2975–2985.
Zalucky AA, Nicholl DD, Hanly PJ, Poulin MJ, Turin TC, Walji S, Handley GB, Raneri JK, Sola DY, Ahmed SB . Nocturnal hypoxemia severity and renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 2015; 192: 873–880.
Nicholl DD, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY, Ahmed SB . Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 2014; 190: 572–580.
Wen ZZ, Cai MY, Mai Z, Jin DM, Chen YX, Huang H, Geng DF, Wang JF . Angiotensin II receptor blocker attenuates intrarenal renin-angiotensin-system and podocyte injury in rats with myocardial infarction. PLoS ONE 2013; 14: e67242.
Fuwa D, Fukuda M, Ogiyama Y, Sato R, Mizuno M, Miura T, Abe-Dohmae S, Michikawa M, Kobori H, Ohte N . Addition of hydrochlorothiazide to angiotensin receptor blocker therapy can achieve a lower sodium balance with no acceleration of intrarenal renin angiotensin system in patients with chronic kidney disease. J Renin Angiotensin Aldosterone Syst 2016; 9: 1470320316652032.
Nishijima Y, Kobori H, Sofue T, Kaifu K, Moriwaki K, Hara T, Hitomi H, Kohno M, Nishiyama A . Important aspects of urine sampling for angiotensinogen measurement: time and preservation conditions in healthy individuals. Tohoku J Exp Med 2012; 228: 333–339.
Nishijima Y, Kobori H, Kaifu K, Mizushige T, Hara T, Nishiyama A, Kohno M . Circadian rhythm of plasma and urinary angiotensinogen in healthy volunteers and in patients with chronic kidney disease. J Renin Angiotensin Aldosterone Syst 2014; 15: 505–508.
Isobe S, Ohashi N, Fujikura T, Tsuji T, Sakao Y, Yasuda H, Kato A, Miyajima H, Fujigaki Y . Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol 2015; 19: 231–239.
Cha RH, Lee H, Lee JP, Lim CS, Kim YS, Kim SG . OS 19-06 change of blood pressure pattern and target organ damage in patients with chronic kidney disease: results of the APrODiTe-2 Study. J Hypertens 2016; 34 (Suppl 1): e230.
Bastos J, Filipa S, Joana S, Raquel F, Polonia J . 6a.05: the prognostic value of ambulatory arterial stiffness index as a predictor of cardiovascular events in resistant hypertensive patients? J Hypertens 2015; 33 (Suppl 1): e74.
Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, Kato A, Miyajima H, Fujigaki Y, Nishiyama A, Yasuda H . Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res 2016; 39: 312–320.
Huang Y, Yamamoto T, Misaki T, Suzuki H, Togawa A, Ohashi N, Fukasawa H, Fujigaki Y, Ichihara A, Nishiyama A, Senbonmatsu T, Ikegaya N, Hishida A . Enhanced intrarenal receptor-mediated prorenin activation in chronic progressive anti-thymocyte serum nephritis rats on high salt intake. Am J Physiol Renal Physiol 2012; 303: F130–F138.
Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I . Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 2012; 23: 1181–1189.
Anderson S, Meyer TW, Rennke HG, Brenner BM . Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 1985; 76: 612–619.
Olson JL, Hostetter TH, Rennke HG, Brenner BM, Venkatachalam MA . Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int 1982; 22: 112–126.
Griffin KA, Picken MM, Bidani AK . Deleterious effects of calcium channel blocker on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest 1995; 96: 793–800.
Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I . Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int 2014; 85: 1068–1077.
Coffman TM, Crowley SD . Kidney in hypertension: guyton redux. Hypertension 2008; 51: 811–816.
Navar LG, Prieto MC, Satou R, Kobori H . Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 2011; 11: 180–186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ohashi, N., Isobe, S., Ishigaki, S. et al. Circadian rhythm of blood pressure and the renin–angiotensin system in the kidney. Hypertens Res 40, 413–422 (2017). https://doi.org/10.1038/hr.2016.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.166
Keywords
This article is cited by
-
Postoperative Dipping Patterns of Mean Arterial Pressure and Mortality After Coronary Artery Bypass Grafting
Journal of Cardiovascular Translational Research (2024)
-
Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases
Journal of Human Hypertension (2022)
-
A disturbance beyond the barrier—chronic kidney disease allows angiotensinogen invasion
Hypertension Research (2021)
-
Circadian rhythm of the intrarenal renin–angiotensin system is caused by glomerular filtration of liver-derived angiotensinogen depending on glomerular capillary pressure in adriamycin nephropathy rats
Hypertension Research (2021)
-
Increased fibroblast growth factor-21 in chronic kidney disease is a trade-off between survival benefit and blood pressure dysregulation
Scientific Reports (2019)