Abstract
There is a deep relationship between cardiovascular disease and periodontitis. It has been reported that myocardial hypertrophy may be affected by periodontitis in clinical settings. Although these clinical observations had some study limitations, they strongly suggest a direct association between severity of periodontitis and left ventricular hypertrophy. However, the detailed mechanisms between myocardial hypertrophy and periodontitis have not yet been elucidated. Recently, we demonstrated that periodontal bacteria infection is closely related to myocardial hypertrophy. In murine transverse aortic constriction models, a periodontal pathogen, Aggregatibacter actinomycetemcomitans markedly enhanced cardiac hypertrophy with matrix metalloproteinase-2 activation, while another pathogen Porphyromonas gingivalis (P.g.) did not accelerate these pathological changes. In the isoproterenol-induced myocardial hypertrophy model, P.g. induced myocardial hypertrophy through Toll-like receptor-2 signaling. From our results and other reports, regulation of chronic inflammation induced by periodontitis may have a key role in the treatment of myocardial hypertrophy. In this article, we review the pathophysiological mechanism between myocardial hypertrophy and periodontitis.
Similar content being viewed by others
Introduction
There is a deep relationship between cardiovascular disease and periodontitis; however, the detailed mechanisms between myocardial hypertrophy and periodontitis have not yet been elucidated.1 Recently, we demonstrated that periodontal bacteria infection accelerated myocardial hypertrophy.2, 3, 4 Chronic inflammation induced by periodontitis may have a key role in the development of myocardial hypertrophy. In this article, we review the pathophysiological mechanism between myocardial hypertrophy and periodontitis.
Myocardial hypertrophy and chronic inflammation
Among the various cardiovascular diseases, myocardial hypertrophy is the most common manifestation because hypertension and other systemic diseases induce the condition.5 It is recognized that chronic inflammation induced by infection could be a cause of myocardial hypertrophy. HIV infection is the most well-known pathogenesis of myocardial hypertrophy. Kearney et al.6 revealed that more than half of the children with HIV infection had myocardial hypertrophy. This was proved by echocardiogram and postmortem cardiac pathology. Similarly, Georgescu et al.7 showed that 25.6% of child patients with HIV infection had left ventricular (LV) myocardial hypertrophy judged by echocardiogram. Breuckmann et al.8 also revealed that a quarter of patients with HIV infection had myocardial hypertrophy. This evaluation was performed by MRI. At this moment, there is no clinical report to show the direct relationship between myocardial hypertrophy and chronic inflammation induced by bacterial infection. However, Kita et al.9 showed that Escherichia coli endotoxin enhanced myocardial hypertrophy in rats with chronic alcohol consumption. Thus, chronic inflammation induced by bacterial infection may be a cause of myocardial hypertrophy. Persistent inflammation has a pathogenic role in chronic heart failure by influencing heart contractility, inducing hypertrophy and promoting apoptosis, which contributes to myocardial remodeling.10, 11 It was also reported that the heart failure with preserved ejection fraction paradigm shifted from LV afterload excess to coronary microvascular inflammation. The new paradigm consists of a systemic proinflammatory state that causes coronary microvascular endothelial inflammation that reduces nitric oxide bioavailability, cyclic guanosine monophosphate content and protein kinase G (PKG). Low PKG activity enhances myocardial hypertrophy development because of hypophosphorylation of titin.12 Biwer et al.13 showed the temporal response to nitric oxide synthase (NOS) and renin-angiotensin system inhibition with respect to cardiac hypertrophy in rats. They demonstrated that L-NAME with enalapril accelerated a significant increase in cardiac mass in the spontaneously hypertensive rat (SHR). Thus, NOS and renin-angiotensin system also has critical roles in cardiac hypertrophy. Nakashima et al.14 showed that reactive oxygen species are critical in angiotensin II-induced vascular remodeling. Taken together, several factors are involved in pressure overload-induced cardiovascular remodeling.
Periodontitis is a major cause of chronic inflammation
Many researchers have studied the relationship between periodontitis and systemic diseases, because periodontitis is a major cause of systemic and chronic inflammation.15, 16 Because a relationship between periodontal disease and cardiovascular disease has been broadly recognized,17 we investigated the pathophysiological relationship between periodontitis and cardiovascular disease.18, 19 Periodontitis is an infectious disease induced by many species of periodontal bacteria, such as Porphyromonas gingivalis (P.g.), Aggregatibacter actinomycetemcomitans (A.a.) and Prevotella intermedia.20 It was reported that each periodontal pathogen influenced the progression of abdominal aortic aneurysm.21, 22, 23 We also reported that P.g. accelerated the progression of abdominal aortic aneurysm through Toll-like receptors (TLRs) and matrix metalloproteinases (MMPs) using experimental murine models.24, 25, 26 On the other hand, there are limited clinical and experimental reports revealing the relationship between periodontitis and myocardial hypertrophy at this point. Moreover, the clinical influence of specific periodontal bacterium on myocardial hypertrophy has not been clarified epidemiologically.
Myocardial hypertrophy may be affected by periodontitis in clinical settings
Clinical observations revealed a relationship between myocardial hypertrophy and periodontitis. The Hisayama study showed that mean probing depth, mean attachment loss, number of teeth and plaque index were associated with LV hypertrophy. In multivariate analysis, the subjects with deep pocket depth had an increased risk of LV hypertrophy compared with the subjects without deep pocket depth. Subjects with severe attachment loss also had significant risk of LV hypertrophy. Although this observation clearly showed the relationship between LV hypertrophy and periodontitis, it has a study limitation because they only judged LV hypertrophy using electrocardiogram (ECG).27 Angeli et al.28 showed a clinical association between periodontitis and LV mass in subjects with essential hypertension. LV mass progression was dependent on the severity of periodontitis. Body surface area, systolic and diastolic blood pressure, and LV mass were determinants of a composite of severe periodontitis. In a multivariate logistic analysis, LV mass was the only determinant of severe periodontitis. Their findings suggested a direct association between the severity of periodontitis and increased LV mass in subjects with essential hypertension. Although they measured LV mass using echocardiogram, this paper has a study limitation in the diagnosis of periodontitis. They evaluated periodontitis using only the community periodontal index of treatment needs (CPITN). CPITN is a convenient methodology to diagnose the severity of periodontits, for example, CPITN 0 (periodontal health), CPITN 1 (gingival bleeding), CPITN 2 (calculus), CPITN 3 (pockets 4–5 mm) and CPITN 4 (pockets ⩾6 mm), it lacks other periodontal information such as attachment loss, number of teeth, and plaque index.28 Franek et al.29 also demonstrated an association between chronic periodontitis and increased LV mass in subjects with Type 2 diabetes mellitus. They clarified that subjects with periodontitis had larger LV mass compared with non-periodontitis subjects. They concluded that periodontitis was associated with increased LV mass and elevated central and systemic blood pressure in subjects with Type 2 diabetes. This study clearly demonstrated the relationship between precisely measured LV mass and periodontitis. However, periodontitis was judged as three biofilm-gingival interface groups; healthy, gingivitis and periodontitis using semi-quantitative methods. Thus, lacking enough information of periodontitis and patients enrollment only from Type 2 diabetes are the study limitation of this paper.29 Although these clinical observations had some study limitations, they strongly suggest a direct association between severity of periodontitis and LV hypertrophy. (Table 1)
Specific periodontal pathogens deteriorate load-induced myocardial hypertrophy
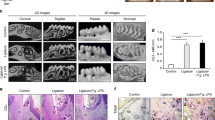
We demonstrated that some specific periodontal pathogens accelerated myocardial hypertrophy. First, we showed that a major periodontal pathogen deteriorated pressure overload-induced myocardial hypertrophy in mice. To establish myocardial hypertrophy in an animal model, transverse aortic constriction (TAC) was performed in mice. We injected a periodontal pathogen, A.a. in the infected group and phosphate buffered saline (PBS) in the control group. We showed that heart per body weight ratio increased in the A.a. infected group compared with the control group. Histopathologically, A.a.-infected mice showed markedly enhanced cardiac hypertrophy, fibrosis and arteriosclerosis 4 weeks after TAC operation. Immunohistochemistry revealed that expression of MMP-2 in the interstitial tissue was enhanced in the A.a.-infected group.2 Pressure overload-induced myocardial hypertrophy is known to be caused by changes in cardiac myocytes and abnormalities in the extracellular matrix network. Progressive LV remodeling and extracellular matrix degradation is associated with increased MMP activity. It is also well recognized that periodontal pathogens increase the activity of MMPs. The activation of MMP-2 was also induced by the lipopolysaccharides of A.a.30 MMP-2 is involved in physiological tissue remodeling and pathological extracellular matrix degradation in the pathogenesis of periodontal diseases.31 Thus, our results suggest that a periodontal pathogen causes a deterioration of pressure overload-induced myocardial hypertrophy through MMP-2 activation. We also evaluated the effect of another periodontal pathogen, P.g. on TAC-induced myocardial hypertrophy. Interestingly, P.g. worsened area of myocardial fibrosis, while the degree of hypertrophy was comparable between the P.g.-infected group and the control group.3 At this moment, the reason why the different pathogens showed different results is to be clarified. It is known that A.a. secretes leukotoxin, which affects polymorphonuclear leukocytes, lymphocytes and macrophages via TLR2 and TLR4. If these cells are activated, proinflammatory cytokines are produced, and it results in enhanced inflammation. On the other hand, P.g. secretes gingipine which works to degrade cytokines, thereby downregulating the host response in the form of reduced inflammation through TLR2 signaling.32, 33, 34, 35 Therefore, the effect of periodontal pathogens is varied on the progression of myocardial hypertrophy induced by pressure overload.
A periodontal pathogen accelerates isoproterenol-induced myocardial hypertrophy
Next, we observed the effect of a periodontal pathogen on catecholamine induced myocardial hypertrophy. To make the model, we subcutaneously implanted a coil-shaped chamber into the back of a mouse and P.g. was injected into the chamber. Following this, an osmotic pump was implanted to infuse isoproterenol systemically. Four weeks after the isoproterenol infusion, we performed an echocardiography and harvested the hearts and blood. Microscopically, we found stronger cardiomyocyte hypertrophy in P.g.-infected mice compared with the control mice. We also detected a higher level of mRNA expression in TLR2 and NADPH oxidase 4 (Nox4) in P.g.-infected mice compared with the control mice.4 It is well known that P.g. enhanced the expression of TLR2 in various kinds of cells. Because we detected a higher expression of TLR2 in P.g.-injected mice, it implied that P.g. enhanced the expression of TLR2 in the hearts. Regarding cardiac hypertrophy, a study using the TAC model reported that the hypertrophy was suppressed in TLR2-deficient mice.36 These results and our observation suggest that TLR2 may have a critical role in cardiomyocyte hypertrophy induced by periodontal pathogens. It was also reported that P.g. enhanced the expression of Nox4 in some cells. Nox4 is known to have an important role in cardiomyocyte hypertrophy. Upregulation of Nox4 in the myocardium causes cardiac remodeling through the activation of Akt-mTOR and NF-κB signaling pathways.37 Therefore, P.g. infection enhances Nox4, resulting in cardiomyocyte hypertrophy. These results suggest that a periodontal pathogen effects isoproterenol-induced cardiac hypertrophy via oxidative stress.
Possible pathophysiological mechanisms between periodontitis and myocardial hypertrophy
The systemic inflammatory state induced by periodontal pathogens affects the human coronary endothelium.38, 39 Proinflammatory cytokines (for example, interleukin-6, tumor necrosis factor-alpha) induce vascular cell adhesion molecule-1 and E-selectin expression on the endothelium.40 Their expression leads to activation and subendothelial migration of circulating leukocytes.41 These cytokines also induce endothelial production of reactive oxygen species through activation of nicotinamide adenine dinucleotide phosphate oxidases.42 Production of reactive oxygen species leads to formation of peroxynitrite (ONOO-) and reduced nitric oxide (NO) bioavailability, both suppress soluble guanylate cyclase activity in adjacent cardiomyocytes. NO is also known to contribute to pathological effects of periodontitis in the heart.43 Lower soluble guanylate cyclase activity decreases cyclic guanosine monophosphate concentration and PKG activity. Low PKG activity increases resting tension of cardiomyocytes because of hypophosphorylation of titin and removes the brake on prohypertrophic stimuli, inducing cardiomyocyte hypertrophy.12 The PKG function as a brake on myocardial hypertrophy has been observed in a wide variety of experimental and clinical settings. In cardiomyocytes cultured from neonatal rat hearts, NO or a cyclic guanosine monophosphate analog attenuated the norepinephrine-induced hypertrophic response.44 Ruiz-Hurtado et al.45 showed that LA-419, which protected NO from degradation, has been found to restore the complete NO signaling cascade and reduce LV remodeling in a model of aortic stenosis-induced pressure overload. Because LA-419 did not restore the original pressure gradient, the increasing NO bioavailability has a possible direct anti-proliferative effect on cardiac myocytes. In mice subjected to TAC, sildenafil, which increases myocardial PKG activity through inhibited breakdown of cyclic guanosine monophosphate by phosphodiesterase 5 (PDE5), prevented or reversed cardiomyocyte hypertrophy by deactivating multiple prohypertrophic pathways.46 In patients with diabetic cardiomyopathy and concentric LV remodeling, sildenafil treatment reduced the LV mass/volume ratio.47 In line with these experimental and clinical findings, lower myocardial PKG activity was shown to correlate with a larger cardiomyocyte diameter. A similar relationship between myocardial PKG activity and cardiomyocyte hypertrophy was also present in aortic stenosis patients who had less myocardial PKG activity and more cardiomyocyte hypertrophy.48 We demonstrated the hypothetical idea with representative factors which have a relationship between periodontal pathogen-induced chronic inflammation and cardiomyocyte hypertrophy (Figure 1).
Possible mechanisms between periodontitis and myocardial hypertrophy. cGMP, cyclic guanosine monophosphate; IL, interleukin; MMPs, matrix metalloproteinases; NF-κB, nuclear factor-kappa B; NO, nitric oxide; Nox, nicotinamide adenine dinucleotide phosphate oxidase; ONOO, peroxynitrite; PKG, protein kinase G; ROS, reactive oxygen species; sGC, soluble guanylate cyclase; TNF, tumor necrosis factor; TLRs, Toll-like receptors; VCAMs, vascular cell adhesion molecules.
In conculsion, periodontitis contributes to a systemic inflammatory state, which induces oxidative stress in the coronary microvascular endothelium. This reduces myocardial NO bioavailability and leads to reduced PKG activity in cardiomyocytes, which enhances myocardial hypertrophy. The new paradigm between periodontitis and myocardial hypertrophy has important diagnostic and therapeutic implications.
References
Suzuki J, Aoyama N, Ogawa M, Hirata Y, Izumi Y, Nagai R, Isobe M . Periodontitis and cardiovascular diseases. Expert Opin Ther Targets 2010; 14: 1023–1027.
Sekinishi A, Suzuki J, Aoyama N, Ogawa M, Watanabe R, Kobayashi N, Hanatani T, Ashigaki N, Hirata Y, Nagai R, Izumi Y, Isobe M . A periodontal pathogen Aggregatibacter actinomycetemcomitans deteriorates pressure overload-induced myocardial hypertrophy in mice. Int Heart J 2012; 53: 324–330.
Kaneko M, Suzuki J, Aoyamay N, Watanabe R, Yoshida A, Shiheido Y, Izumi Y, Isobe M . Toll-like receptor-2 in periodontal pathogen-induced myocardial fibrosis in pressure overloaded murine hearts. Hypertens Res (e-pub ahead of print 1 September 2016; doi:10.1038/hr.2016.117).
Sato H, Suzuki J, Aoyama N, Watanabe R, Kaneko M, Shiheido Y, Yoshida A, Wakayama K, Kumagai H, Ikeda Y, Akazawa H, Komuro I, Isobe M, Izumi Y . A Periodontal pathogen Porphyromonas gingivalis deteriorates Isoproterenol-Induced myocardial remodeling in mice. Hypertens Res (e-pub ahead of print 8 September 2016; doi:10.1038/hr.2016.114).
Oka T, Akazawa H, Naito AT, Komuro I . Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014; 114: 565–571.
Kearney DL, Perez-Atayde AR, Easley KA, Bowles NE, Bricker JT, Colan SD, Kaplan S, Lai WW, Lipshultz SE, Moodie DS, Sopko G, Starc TJ, Towbin JA . Postmortem cardiomegaly and echocardiographic measurements of left ventricular size and function in children infected with the human immunodeficiency virus. The Prospective P2C2 HIV Multicenter Study. Cardiovasc Pathol 2003; 12: 140–148.
Georgescu AM, Moldovan C, Azamfirei L, Georgescu D . Ultrasound and histopathological features of myocardial involvement in HIV infection in children. Rom J Morphol Embryol 2014; 55: 773–779.
Breuckmann F, Nassenstein K, Kondratieva J, Esser S, Erbel R, Potthoff A, Brockmeyer NH, Neumann T, Barkhausen J . MR characterization of cardiac abnormalities in HIV+ individuals with increased BNP levels. Eur J Med Res 2007; 12: 185–190.
Kita T, Nagano T, Kasai K, Tanaka N . E. coli endotoxin enhances cardiomyopathy in rats with chronic alcohol consumption. Int J Legal Med 1996; 109: 37–41.
Aukrust P, Yndestad A, Damås JK, Gullestad L . Inflammation and chronic heart failure-potential therapeutic role of intravenous immunoglobulin. Autoimmun Rev 2004; 3: 221–227.
Aukrust P, Gullestad L, Ueland T, Damås JK, Yndestad A . Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med 2005; 37: 74–85.
Paulus WJ, Tschouml;pe C . A novel paradigm for heart failure with preserved ejection fraction. J Am Coll Cardiol 2013; 62: 263–271.
Biwer LA, D'souza KM, Abidali A, Tu D, Siniard AL, DeBoth M, Huentelman M, Hale TM . Time course of cardiac inflammation during nitric oxide synthase inhibition in SHR: impact of prior transient ACE inhibition. Hypertens Res 2016; 39: 8–18.
Nakashima T, Umemoto S, Yoshimura K, Matsuda S, Itoh S, Murata T, Fukai T, Matsuzaki M . TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertens Res 2015; 38: 649–655.
Offenbacher S . Periodontal diseases: pathogenesis. Ann Periodontol 1996; 1: 821–878.
Offenbacher S, Barros SP, Beck JD . Rethinking periodontal inflammation. J Periodontol 2008; 79: 1577–1584.
Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC Jr, Baddour LM American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation 2012; 125: 2520–2544.
Suzuki J, Aoyama N, Aoki M, Tada Y, Wakayama K, Akazawa H, Shigematsu K, Hoshina K, Izumi Y, Komuro I, Miyata T, Hirata Y, Isobe M . Incidence of periodontitis in Japanese patients with cardiovascular diseases -a comparison between abdominal aortic aneurysm and arrhythmia. Heart Vessels 2015; 30: 498–502.
Schenkein HA, Loos BG . Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol 2013; 84: S51–S69.
Socransky SS, Haffajee AD . Dental biofilms: difficult therapeutic targets. Periodontol 2000 2002; 28: 12–55.
Kurihara N, Inoue Y, Iwai T, Umeda M, Huang Y, Ishikawa I . Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2004; 28: 553–558.
Paraskevas KI, Mikhailidis DP, Giannoukas AD . Periodontitis and abdominal aortic aneurysms: a random association or a pathogenetic link? Int Angiol 2009; 28: 431–433.
Delbosc S, Alsac JM, Journe C, Louedec L, Castier Y, Bonnaure-Mallet M, Ruimy R, Rossignol P, Bouchard P, Michel JB, Meilhac O . Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS ONE 2011; 6: e18679.
Aoyama N, Suzuki J, Wang D, Ogawa M, Kobayashi N, Hanatani T, Takeuchi Y, Izumi Y, Isobe M . Porphyromonas gingivalis promotes murine abdominal aortic aneurysms via matrix metalloproteinase-2 induction. J Periodontal Res 2011; 46: 176–183.
Aoyama N, Suzuki J, Ogawa M, Watanabe R, Kobayashi N, Hanatani T, Yoshida A, Ashigaki N, Izumi Y, Isobe M . Clarithromycin suppresses the periodontal bacteria-accelerated abdominal aortic aneurysms in mice. J Periodontal Res 2012; 47: 463–469.
Aoyama N, Suzuki J, Ogawa M, Watanabe R, Kobayashi N, Hanatani T, Ashigaki N, Sekinishi A, Izumi Y, Isobe M . Toll-like receptor-2 plays a fundamental role in periodontal bacteria-accelerated abdominal aortic aneurysms. Circ J 2013; 77: 1565–1573.
Shimazaki Y, Saito T, Kiyohara Y, Kato I, Kubo M, Iida M, Koga T . Relationship between electrocardiographic abnormalities and periodontal disease: the Hisayama Study. J Periodontol 2004; 75: 791–797.
Angeli F, Verdecchia P, Pellegrino C, Pellegrino RG, Pellegrino G, Prosciutti L, Giannoni C, Cianetti S, Bentivoglio M . Association between periodontal disease and left ventricle mass in essential hypertension. Hypertension 2003; 41: 488–492.
Franek E, Napora M, Blach A, Budlewski T, Gozdowski D, Jedynasty K, Krajewski J, Gorska R . Blood pressure and left ventricular mass in subjects with type 2 diabetes and gingivitis or chronic periodontitis. J Clin Periodontol 2010; 37: 875–880.
Tiranathanagul S, Yongchaitrakul T, Pattamapun K, Pavasant P . Actinobacillus actinomycetemcomitans lipopolysaccharide activates matrix metalloproteinase-2 and increases receptor activator of nuclear factor-kappaB ligand expression in human periodontal ligament cells. J Periodontol 2004; 75: 1647–1654.
Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P . Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 2006; 38: 306–321.
Sun Y, Shu R, Li CL, Zhang MZ . Gram-negative periodontal bacteria induce the activation of Toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol 2010; 81: 1488–1496.
Burns E, Eliyahu T, Uematsu S, Akira S, Nussbaum G . TLR2-dependent inflammatory response to Porphyromonas gingivalis is MyD88 independent, whereas MyD88 is required to clear infection. J Immunol 2010; 184: 1455–1462.
Stathopoulou PG . Epithelial cell pro-inflammatory cytokine response differs across dental plaque bacterial species. J Clin Periodontol 2010; 37: 24–29.
Olsen I, Shah HN, Gharbia SE . Taxonomy and biochemical characteristics of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontol 2000 1999; 20: 14–52.
Higashikuni Y, Tanaka K, Kato M, Nureki O, Hirata Y, Nagai R, Komuro I, Sata M . Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1b upregulation via nuclear factor κB activation. J Am Heart Assoc 2013; 2: e000267.
Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, Abboud HE . NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NF-κB signaling pathways. Circulation 2015; 131: 643–655.
Honda T, Oda T, Yoshie H, Yamazaki K . Effects of Porphyromonas gingivalis antigens and proinflammatory cytokines on human coronary artery endothelial cells. Oral Microbiol Immunol 2005; 20: 82–88.
Yun PL, Decarlo AA, Hunter N . Gingipains of Porphyromonas gingivalis modulate leukocyte adhesion molecule expression induced in human endothelial cells by ligation of CD99. Infect Immun 2006; 74: 1661–1672.
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C . Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 2011; 4: 44–52.
Van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ . Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008; 117: 43–51.
Griendling KK, Sorescu D, Ushio-Fukai M . NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000; 86: 494–501.
Herrera BS, Martins-Porto R, Campi P, Holzhausen M, Teixeira SA, Mendes GD, Costa SK, Gyurko R, Van Dyke TE, Spolidório LC, Muscará MN . Local and cardiorenal effects of periodontitis in nitric oxide-deficient hypertensive rats. Arch Oral Biol 2011; 56: 41–47.
Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS . Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest 1998; 101: 812–818.
Ruiz-Hurtado G, Delgado C . Nitric oxide pathway in hypertrophied heart: new therapeutic uses of nitric oxide donors. J Hypertens 2010; 28: S56–S61.
Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA . Chronic inhibition of cyclic GMP phosphodiesterase 5 A prevents and reverses cardiac hypertrophy. Nat Med 2005; 11: 214–222.
Giannetta E, Isidori AM, Galea N, Carbone I, Mandosi E, Vizza CD, Naro F, Morano S, Fedele F, Lenzi A . Chronic inhibition of cGMP phosphodiesterase 5 A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation 2012; 125: 2323–2333.
Falcão-Pires I, Hamdani N, Borbély A, Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen GJ, Niessen HW, Leite-Moreira AF, Paulus WJ . Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 2011; 124: 1151–1159.
Acknowledgements
We thank Ms Noriko Tamura for excellent contributions. This work was supported by grants from Mitsui Life Insurance Research Foundation, Mitsui Sumitomo Marine Welfare Research Foundation, Geriatric Dental Research Foundation, Human Health Future Research Foundation, St Luke’s Hospital Research Foundation, Health Management Foundation, Taiyo Life Insurance Research Foundation, The 8020 Promotion Foundation, Terumo Science Foundation, Pfizer Health Research Foundation, General Health Promotion Foundation, Suzuken Memorial Foundation, Health Science Center Foundation, Kobayashi International Scholarship Foundation, Hakujikai Institute of Gerontology Foundation and the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K20616 and 16H05824.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JS received research funding of 2 000 000 yen or more in 1 year. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Suzuki, Ji., Sato, H., Kaneko, M. et al. Periodontitis and myocardial hypertrophy. Hypertens Res 40, 324–328 (2017). https://doi.org/10.1038/hr.2016.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.146
Keywords
This article is cited by
-
Echocardiographic Evaluation of Healthy Subjects with Different Grades of Periodontal Disease
SN Comprehensive Clinical Medicine (2022)
-
Longitudinal changes of cardiac troponin and inflammation reflect progressive myocyte stretch and likelihood for hypertension in a Black male cohort: The SABPA study
Hypertension Research (2019)