Abstract
Our earlier studies of preeclampsia (PE) at delivery have demonstrated the alteration of one carbon cycle, reduced placental omega 3 fatty acids, altered circulating levels of angiogenic factors and differential placental gene-specific methylation patterns of angiogenic factors. This study was undertaken to examine changes in the levels of angiogenic factors and angiotensin II type 1 receptor autoantibodies (AT1-AAs) throughout gestation, from early pregnancy until delivery, in women with PE and to examine their association with cord angiogenic factors, blood pressure and infant weight. A total of 81 pregnant women (46 normotensive and 35 with PE) were followed at three different time points during pregnancy: 16–20 weeks (T1), 26–30 weeks (T2) and at the time of delivery (T3). The plasma levels of angiogenic factors and AT1-AAs were determined in the maternal and cord plasma by commercial enzyme-linked immunosorbent assay kits. Maternal plasma levels of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) were lower (P<0.05 for both), whereas soluble fms-like tyrosine kinase-1 (sFlt-1; P<0.05) and the sFlt-1/PlGF ratio (P<0.01) were higher in early pregnancy in the PE group. Maternal plasma AT1-AA levels were higher (P<0.05) at T2 in women with PE. Cord plasma VEGF and soluble kinase insert domain receptor (sKDR) levels were lower (P<0.01 and P<0.05, respectively), whereas AT1-AA levels were higher (P<0.05) in the PE group. Maternal plasma VEGF levels in early pregnancy were positively associated with systolic blood pressure, whereas the sFlt-1/PlGF ratio at T2 was negatively associated with infant weight in the PE group. Low levels of proangiogenic factors (VEGF and PlGF) and high levels of AT1-AAs and antiangiogenic factors (sFlt-1 and sFlt-1/PlGF ratio) are present in the maternal circulation during early gestation in women with PE.
Similar content being viewed by others
Introduction
Preeclampsia (PE), a pregnancy complication affecting 2 to 8% of all pregnancies worldwide,1 is characterized by the onset of hypertension and proteinuria after 20 weeks of gestation.2 The pathogenesis of PE involves inadequate placental vascular remodeling that leads to defective placentation and placental ischemia, resulting in maternal endothelial dysfunction.3, 4, 5 During pregnancy, vasculogenesis and extensive angiogenesis are required for placental and fetal development,6, 7 and any disturbance in these processes may result in an adverse pregnancy outcome. The role of angiogenic proteins such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF) and their receptors, fms-like tyrosine kinase-1 (Flt-1) and kinase insert domain receptor (KDR), in angiogenesis is well described.8 Studies have also reported higher circulating autoantibodies against angiotensin II receptor type 1 (AT1) in women with PE.9, 10 Angiotensin II type 1 receptor autoantibodies (AT1-AAs) have been shown to inhibit trophoblast migration,11 induce reactive oxygen species in vitro12 and induce PE-like symptoms in animal models,13, 14 indicating that these autoantibodies may contribute to the pathophysiology of PE.
Our earlier cross-sectional studies in women with PE indicate altered angiogenesis in the maternal and cord plasma at delivery as well as an association with poor birth outcome.15 These results were observed at the end of pregnancy when the pathology had already progressed. However, it would be highly worthwhile to examine changes in these angiogenic factors in early pregnancy. Furthermore, it has been suggested that changes in the splice variant of Flt-1, known as soluble Flt-1 (sFlt-1), alone or combined with PlGF may be useful biomarkers for the prediction of PE.16, 17 A number of longitudinal studies have examined changes in angiogenic and antiangiogenic factors in women with PE, but the results are inconsistent. Some studies suggest that changes in angiogenic factors across gestation precede the clinical presentation of PE,18, 19 whereas others suggest that the prediction of PE in early pregnancy may not be possible with the use of maternal angiogenic protein levels.20, 21 These inconsistent results may be attributable to the heterogeneous populations included in the studies, with varying ethnicities, socioeconomic status and smoking status,18, 22 all of which are known to affect the levels of angiogenic factors.23, 24 Furthermore, no studies have examined the longitudinal changes across gestation in AT1-AA levels in women with PE.
Recently, we have reported the plasma levels of angiogenic factors throughout gestation and their associations with blood pressure and birth outcome in normotensive pregnancy. Our results indicate that maternal VEGF levels in early gestation (16–20 weeks) play an important role in predicting birth weight in healthy term pregnancies.25 The current study was undertaken to examine changes in the maternal plasma levels of angiogenic (VEGF and PlGF) and antiangiogenic (sFlt-1 and sKDR) factors and AT1-AAs throughout gestation, from early pregnancy until delivery, as well as their association with blood pressure, cord levels at delivery and birth weight in women with PE. The study also examined whether the sFlt-1/PlGF ratio in early pregnancy could be useful in determining the risk for developing PE.
Methods
Subjects
This prospective study was conducted at Gupte Hospital and Research Centre, Pune. The study was approved by the Bharati Vidyapeeth Medical College Institutional Ethics Committee, and written consent was obtained from each subject. This study is part of a large ongoing departmental study that recruits pregnant women at 16–20 weeks of gestation. All these women are followed up until delivery and are categorized as having PE if there is a presence of proteinuria or high blood pressure. PE was defined by systolic and diastolic blood pressures of >140 mm Hg and >90 mm Hg, respectively, with the presence of proteinuria (41+ or 300 mg per 24 h) on a dipstick test. Blood pressure was measured with a mercury sphygmomanometer, and PE was confirmed by repeated recording of the blood pressure with an interval of 6 h at the time of diagnosis. A total number of 81 women with singleton pregnancies (46 normotensive and 35 with PE) were included in this study. Women were excluded from the study if there was evidence of other pregnancy complications such as chronic hypertension, type I or type II diabetes mellitus, seizure disorder or renal or liver disease. All study participants neither consumed alcohol nor smoked that was confirmed when the women were interviewed for their demographic and nutritional history. All women were routinely given iron tablets, folic acid, calcium and multivitamin tablets.
Data for the mothers and blood samples were collected at three different time points: the first time point was at 16–20 weeks (T1), the second time point was at 26–30 weeks (T2), and the third time point was at the time of delivery (T3). The umbilical cord (T4) was also collected just after delivery. Our study design has been previously reported by us in normotensive women.25 Among the 46 normotensive women and 35 women with PE who were enrolled, the first time point blood sample was obtained from 42 normotensive women and 35 women with PE, the second time point blood sample was obtained from 43 normotensive women and 25 women with PE and the delivery sample was obtained from 45 normotensive women and 24 women with PE. An umbilical cord blood sample was obtained from 46 normotensive women and 34 women with PE.
Sample collection and processing
At every time point, 10 ml of maternal venous blood was collected, and umbilical cord blood was collected just after delivery; all samples were collected into ethylenediaminetetraacetic acid-containing vials and were processed as previously reported by us.25
Fetal growth measures
Infant weight, length and head and chest circumference were recorded within half an hour after birth according to standard methods, as reported previously.25
VEGF, PlGF, sFlt-1, sKDR and AT1-AA levels from maternal and cord plasma
Plasma VEGF, PlGF sFlt-1 and sKDR levels were measured in both maternal samples and cord samples using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA), and the procedures have previously been described by us.15 For the sFlt-1 and sKDR assay, plasma samples were diluted 1:5 (vol/vol) in calibrator diluent before following the remaining assay procedure of the manufacturer. Plasma AT1-AA levels were measured from both maternal and cord samples using an ELISA kit according to the manufacturer’s instructions (MyBioSource, San Diego, CA, USA). The detection limit (sensitivity) of the assay was 9 pg ml−1 for VEGF, 7 pg ml−1 for PlGF, 3.5 pg ml−1 for sFlt-1, 4.6 pg ml−1 for sKDR and 0.1 ng ml−1 for AT1-AA. The number of samples analyzed for the parameters at different time points is given in the flow chart (Figure 1).
Flow chart for samples analyzed at different time points. AT1-AAs, angiotensin II type 1 receptor autoantibodies; N, normotensive control; PE, preeclampsia; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; sKDR, soluble kinase insert domain receptor; VEGF, vascular endothelial growth factor.
Statistical analysis
Values are reported as mean±s.e. The data were analyzed using the SPSS/PC+ package (Version 20, Chicago, IL, USA). The data were checked for normal distribution by testing for skewness and kurtosis. Skewed variables were log transformed for normalization. The mean values of the estimates of various parameters for the maternal and cord samples were compared using unadjusted independent sample t-tests to identify statistically significant differences (P<0.05). For each time point, we assessed the association of circulating angiogenic and antiangiogenic factors with the mother’s systolic and diastolic blood pressures, cord levels and with infant weight. The extent of the linear relationship between the angiogenic factors and blood pressure was studied using bivariate correlation analysis. Correlations of angiogenic factors and AT1-AAs with fetal growth measures were examined using partial correlation analysis after adjusting for age, body mass index and gestational age at the time of blood sampling.
Results
Maternal and neonatal demographic characteristics of normotensive women and women with PE
Table 1 shows the maternal and neonatal demographic characteristics of normotensive women and women with PE.
Maternal and cord plasma levels of VEGF, sFlt-1, PlGF, sKDR and AT1-AA and the sFlt-1/PlGF ratio
VEGF
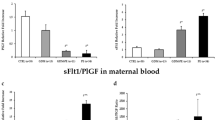
Maternal plasma VEGF levels were lower at T1 (P<0.05), T2 (P<0.05) and T3 (P<0.05) in women with PE as compared with normotensive women. Cord plasma VEGF levels were also lower (P<0.01) in women with PE compared with normotensive women (Figure 2).
Comparison of maternal and cord plasma VEGF, PlGF, sFLT-1, sKDR levels and sFlt-1/PlGF ratio between normotensive and preeclampsia groups. *P<0.05 and **P<0.01 as compared with normotensive women. N, normotensive control; PE, preeclampsia; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; sKDR, soluble kinase insert domain receptor; T1, 16–20 weeks; T2, 26–30 weeks; T3, at the time of delivery; T4, cord; VEGF, vascular endothelial growth factor.
PlGF
Maternal plasma PlGF levels were lower at T1 (P<0.05), T2 (P<0.01) and T3 (P<0.05) in women with PE as compared with normotensive women. Cord plasma PlGF levels were below the detection limit in both groups (Figure 2).
sFlt-1
Maternal plasma sFlt-1 levels were higher at T1 (P<0.05), T2 (P<0.01) and T3 (P<0.05) in women with PE as compared with normotensive women (Figure 2).
sKDR
Maternal plasma sKDR levels were lower at T2 (P<0.05) and T3 (P<0.05) in women with PE as compared with normotensive women. In addition, cord plasma sKDR levels were also lower in women with PE as compared with normotensive women (P<0.05; Figure 2).
sFlt-1/PlGF ratio
The maternal plasma sFlt-1:PlGF ratio was higher at T1 (P<0.01), T2 (P<0.01) and T3 (P<0.05) in women with PE as compared with normotensive women (Figure 2).
AT1-AAs
Maternal plasma AT1-AA levels were higher at T2 (P<0.05) in women with PE as compared with normotensive women. Maternal plasma AT1-AA levels were also higher at T1 and T3 in women with PE, but the differences were not statistically significant. In addition, cord plasma AT1-AA levels were also higher in women with PE as compared with normotensive women (P<0.05; Figure 3).
Comparison of maternal and cord plasma AT1-AA levels between normotensive and preeclampsia groups. *P<0.05 as compared with normotensive women. AT1-AAs, angiotensin II type 1 receptor autoantibodies; N, normotensive control; PE, preeclampsia; T1, 16–20 weeks; T2, 26–30 weeks; T3, at the time of delivery; T4, cord.
Associations of plasma VEGF, PlGF, sFlt-1, AT1-AA and the sFlt-1/PlGF ratio with blood pressure
There was a positive association between maternal plasma VEGF levels and systolic blood pressure (r=0.423, P=0.035, n=25) at T1 in the PE group. The sFlt-1/PlGF ratio at T3 was positively associated with systolic blood pressure in the PE group (r=0.485, P=0.049, n=17). Furthermore, there was a trend toward a positive association of maternal plasma AT1-AA levels with diastolic blood pressure at T1 in the PE group (r=0.371, P=0.057, n=27).
Associations of the maternal plasma sFlt-1/PlGF ratio with infant weight
The maternal plasma sFlt-1/PlGF ratio at T2 was negatively associated with infant weight (r=−0.501, P=0.029, n=21) in women with PE.
Associations of maternal plasma AT1-AA with fetal growth measures
Maternal plasma AT1-AA at T1 was negatively associated with infant length (r=−0.525, P=0.030, n=15) in women with PE. Similarly, maternal plasma AT1-AA at T3 was negatively associated with infant head and chest circumferences (r=−0.580, P=0.007, n=18 and r=−0.486, P=0.030, n=18, respectively) in women with PE.
Discussion
The key findings of our study in women with PE are as follows: (1) reduced maternal plasma VEGF and PlGF levels but increased sFlt-1 levels and an increased sFlt-1/PlGF ratio in early gestation that continued until delivery; (2) reduced maternal plasma sKDR levels at T2, T3 and T4; (3) increased maternal plasma AT1-AA levels at T2; (4) reduced cord plasma VEGF and sKDR and increased cord plasma AT1-AA levels; (5) a positive association between maternal plasma VEGF levels and systolic blood pressure at T1; (6) a negative association between the maternal sFlt-1/PlGF ratio and infant weight at T2; and (7) a negative association between maternal plasma AT1-AAs and fetal growth measures at T1 and T3.
VEGF followed a bell-shaped curve, increasing from the first to second time point and then decreasing from the second to the third time point. The cord plasma VEGF levels were higher when compared with the maternal levels. This could be because of an increased requirement of VEGF by the fetus that can be explained by the ischemic/hypoxic environment present during the first trimester that triggers an increase in VEGF levels to meet the increased metabolic demand of the mother for placental growth and development.26 Our results are consistent with other studies that have reported decreased levels of VEGF in women with PE as compared with normotensive women,19, 27 indicating that VEGF is an important factor involved in the pathogenesis of PE and its complications. Our findings have implications because VEGF is one of the most important growth factors regulating angiogenesis in the endothelium.28
We observed a dramatic increase in the PlGF levels from the first to second time point that then decreased dramatically at the third time point. The decreased PlGF at the third time point may be due to the increased sFlt-1 levels. The decrease in PlGF from the second to third time point and the increase in sFlt-1 from the second to third time point resulted in a much higher sFlt-1/PlGF ratio as compared with the second time point. A well-established placental circulation may explain the observation of the highest levels of PlGF at the second time point. We also observed that maternal plasma PlGF levels were reduced in women with PE as compared with normotensive women at all time points. This is consistent with a number of previous studies.13, 23, 24, 29 It is suggested that reduced PlGF levels early in pregnancy in women who later develop PE could be either because of lower production of PlGF by the placenta, which is smaller in women with PE, or increased binding to receptors in the fetal-placental circulation.19, 30 Furthermore, it is suggested that lower levels of PlGF during early gestation may indicate a greater risk of early-onset PE.19
We observed higher levels of maternal plasma sFlt-1 in women with PE as compared with normotensive women at all time points. Our results are consistent with other reports that suggest that PE is associated with an antiangiogenic state, with high plasma levels of sFlt-1.18, 22, 31, 32, 33, 34 Furthermore, it is proposed that high maternal sFlt-1 levels may induce a systemic endothelial dysfunction that ultimately results in hypertension and proteinuria35 that characterize women with PE.
We and others have reported that in a normotensive pregnancy, the maternal sFlt-1 levels are stable during early and mid-gestation, followed by a steady increase at the time of delivery. Furthermore, it is reported that during the second trimester, the maternal PlGF levels are high and the maternal sFlt-1 levels are low, creating a proangiogenic state.19, 25 In women with PE, sFlt-1 appears to increase earlier in gestation and reach a higher level than it does in controls, possibly leading to altered placental growth and function.
Although the role of sFlt-1 in PE is well established, very little is known about the role of sKDR in PE. We observed lower levels of sKDR in women with PE as compared with normotensive women, indicating that PE is associated with decreased plasma levels of sKDR. Recent studies have also reported lower maternal plasma levels of sKDR during early PE or pregnancies with intrauterine growth restriction.36, 37, 38, 39 The low circulating levels of sKDR could be either because of a decrease in the availability of free VEGF to stimulate KDR synthesis and trafficking at the endothelial cell surface or a low regenerative capacity in endothelial cells.36
We observed increased maternal plasma AT1-AA levels at the second time point in women with PE. In addition, the maternal plasma AT1-AA levels were also higher at the first time point and at the time of delivery in women with PE, but the differences were not statistically significant, perhaps because of the small sample size. Recent reports suggest that these autoantibodies may be useful as biomarkers in women with PE.40 In view of this, our findings are important because we observed an increase in maternal AT1-AAs starting in early pregnancy in women with PE. Our findings show changes in the maternal plasma levels of angiogenic factors (VEGF and PlGF) and antiangiogenic factors (sFlt-1 and sKDR) beginning in early pregnancy that may precede the clinical presentation of PE. We have recently reported that altered placental long-chain polyunsaturated fatty acids may alter the membrane lipid fatty acid composition, leading to the increased release of sFlt-1 in circulation; furthermore, we have also discussed the possibility that the maternal sFlt-1/PlGF ratio may be predictive of PE.15, 41 This is in line with findings reported in recent studies22, 42 but is in contrast to other studies indicating that in early pregnancy, this ratio is not useful for the prediction of PE.20 The cord plasma VEGF levels in this study were lower in women with PE compared with normotensive women and this supports the findings of previous studies.43, 44, 45, 46, 47 This is in contrast to earlier studies by us that included the lower socioeconomic class, in whom we reported higher cord plasma VEGF levels.15 Elevated cord plasma VEGF levels have also been reported by others in women with PE.48 Furthermore, Han et al.49 have reported no difference in cord plasma VEGF levels between PE and normotensive groups. In contrast to VEGF, we observed an increasing trend of cord plasma sFlt-1 levels in women with PE that is consistent with a number of earlier reports.45, 46 Furthermore, in the PE group, the cord plasma sKDR levels were reduced and the cord plasma AT1-AA levels were increased compared with those in normotensive women.
In our study, maternal AT1-AA levels were also negatively correlated with fetal growth measures. It may be possible that maternal AT1-AA gets transferred to the fetus via the placenta, thereby affecting the growth and development of the fetus. Thus, the reduced cord plasma VEGF and sKDR levels and the increased cord plasma sFlt-1 and AT1-AA levels in women with PE indicate that these values are characteristic of mothers with PE, in whom they cause endothelial dysfunction and angiogenic disturbance in the fetal compartment that may subsequently affect birth weight. In our study, there was a positive association between maternal plasma VEGF levels and systolic blood pressure at the first time point (16–20 weeks) in the PE group. This could be a result of hypoxia, as it is known that the placenta develops a hypoxic environment during the first trimester and hypoxia has been shown to increase blood pressure in animals.50 Furthermore, placental ischemia is also known to stimulate AT1-AAs in animals, thereby causing hypertension.51
Our study is the first to report a negative association between the maternal sFlt-1/PlGF ratio at the second time point (26–30 weeks) and infant birth weight, suggesting that defective placentation may affect the birth weight of the fetus. It is suggested that sFlt-1 and PlGF have a high sensitivity from the mid-second trimester, and screening for these biomarkers of PE during early pregnancy may help to optimize the time of delivery and reduce the number of preterm births.52
To summarize, our findings suggest that women with PE have low VEGF and PlGF levels, increased AT1-AA and an increased sFlt-1/PlGF ratio beginning in early gestation. Furthermore, screening for these angiogenic factors during early pregnancy may possibly predict infant birth weight in women with PE. Our earlier studies in PE have identified an altered one-carbon metabolism, hyperhomocysteinemia and increased oxidative stress at delivery.53, 54 We have recently reported altered placental VEGF mRNA expression and CpG methylation patterns in women with PE, suggesting that they are influenced by the intrauterine environment.55 Thus, with all this background, our study highlights the key role that altered angiogenesis in early pregnancy plays in the pathophysiology of PE.
References
Ghulmiyyah L, Sibai B . Maternal mortality from preeclampsia/eclampsia. Sem Perinatol 2012; 36: 56–59.
Roberts JM, Gammill HS . Preeclampsia: recent insights. Hypertension 2005; 46: 1243–1249.
Pijnenborg R, Vercruysse L, Hanssens M . The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006; 27: 939–958.
Burton GJ, Woods AW, Jauniaux E, Kingdom JC . Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009; 30: 473–482.
Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP . Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2008; 294: H541–H550.
Smith SK, He Y, Clark DE, Charnock-Jones DS . Angiogenic growth factor expression in placenta. Semin Perinatol 2000; 24: 82–86.
Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD . Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003; 110 (Suppl 1): S10–S18.
Tammela T, Enholm B, Alitalo K, Paavonen K . The biology of vascular endothelial growth factors. Cardiovasc Res 2005; 65: 550–563.
Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modrow S, Wallukat G, Luft FC, Redman CW, Dechend R . Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension 2009; 53: 393–398.
Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T . Relation between circulating angiotensin II type 1 receptor agonistic autoantibodies and soluble fms-like tyrosine kinase 1 in the pathogenesis of preeclampsia. J Clin Endocrinol Metab 2006; 91: 2424–2427.
Xia Y, Wen H, Bobst S, Day MC, Kellems RE . Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig 2003; 10: 82–93.
Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC . AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003; 107: 1632–1639.
Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN . Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension 2005; 45: 742–746.
Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y . Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008; 14: 855–862.
Kulkarni AV, Mehendale SS, Yadav HR, Kilari AS, Taralekar VS, Joshi SR . Circulating angiogenic factors and their association with birth outcomes in preeclampsia. Hypertens Res 2010; 33: 561–567.
Woolcock J, Hennessy A, Xu B, Thornton C, Tooher J, Makris A, Ogle R . Soluble Flt-1 as a diagnostic marker of pre-eclampsia. Aust N Z J Obstet Gynaecol 2008; 48: 64–70.
De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’anna R . Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand 2008; 87: 837–842.
Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA . A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble VEGF receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small-for-gestational-age neonate. J Matern Fetal Neonatal Med 2008; 21: 9–23.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA . Circulating angiogenic factors and the risk of preeclampsia. New Engl J Med 2004; 350: 672–683.
McElrath TF, Lim KH, Pare E, Rich-Edwards J, Troisi R, Parry S . Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol 2012; 207: e1–e7.
Sezer SD, Küçük M, Yenisey C, Yüksel H, Odabaşı AR, Türkmen MK, Çetinkaya Çakmak B, Ömürlü IK . Comparison of angiogenic and anti-angiogenic factors in maternal and umbilical cord blood in early- and late-onset pre-eclampsia. Gynecol Endocrinol 2012; 28: 628–632.
Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA . Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012; 125: 911–919.
Ambrose JA, Barua RS . The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004; 43: 1731–1737.
Mijal RS, Holzman CB, Rana S, Karumanchi SA, Wang J, Siroskii A . Mid pregnancy levels of angiogenic markers in relation to maternal characteristics. Am J Obstet Gynecol 2011; 204: e1–12.
Sundrani D, Khot V, Pisal H, Mehendale S, Wagh G, Joshi A, Joshi S . Gestation dependant changes in angiogenic factors and their associations with fetal growth measures in normotensive pregnancy. PLoS One 2013; 8: e54153.
Adair TH, Gay WJ, Montani JP . Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol 1990; 259: R393–R404.
Livingston JC . Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol 2000; 183: 1554–1557.
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J . Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248.
Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA . First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 2004; 89: 770–775.
Taylor RN, Grimwood J, Taylor RS, Mc- Master MT, Fisher SJ, North RA . Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 2003; 188: 177–182.
Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, August P . Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension 2012; 59: 740–746.
Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC . Changes in circulating levels of angiogenic factors from the first to second trimesters as predictors of preeclampsia. Am J Obstet Gynecol 2007; 196: 239.e1–239.e6.
Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Than NG, Gomez R, Hassan SS . The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008; 21: 279–287.
Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Gonçalves L, Gomez R, Edwin S, Mazor M . Plasma soluble vascular endothelial growth factor receptor-1 concentrations elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 2005; 17: 3–18.
Maynard S, Epstein FH, Karumanchi SA . Preeclampsia and angiogenic imbalance. Annu Rev Med 2008; 59: 61–78.
Munaut C, Lorquet S, Pequeux C, Coulon C, Le Goarant J, Chantraine F, Noël A, Goffin F, Tsatsaris V, Subtil D, Foidart JM . Differential expression of Vegfr-2 and its soluble form in preeclampsia. PLoS One 2012; 7: e33475.
Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D . Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci 2007; 112: 51–57.
Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, Pineles BL, Papp Z, Hassan S . Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med 2008; 21: 41–52.
Tripathi R, Rath G, Ralhan R, Saxena S, Salhan S . Soluble and membranous vascular endothelial growth factor receptor-2 in pregnancies complicated by pre-eclampsia. Yonsei Med J 2009; 50: 656–666.
Xia Y, Kellems RE . Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res 2013; 113: 78–87.
Kulkarni AV, Mehendale SS, Yadav HR, Joshi SR . Reduced placental docosahexaenoic acid levels associated with increased levels of sFlt-1 in preeclampsia. Prostaglandins Leukot Essent Fatty Acids 2011; 84: 51–55.
Leaños-Miranda A, Campos-Galicia I, Isordia-Salas I, Rivera-Leaños R, Romero-Arauz JF, Ayala-Méndez JA, Ulloa-Aguirre A . Changes in circulating concentrations of soluble fms-like tyrosine kinase-1 and placental growth factor measured by automated electrochemiluminescence immunoassays methods are predictors of preeclampsia. J Hypertens 2012; 30: 2173–2181.
Kwon JY, Maen YS, Kwon YG, Kim YH, Kang MH, Park YW . Endothelial progenitor cells in umbilical cord blood in severe preeclampsia. Gynecol Obstet Invest 2007; 64: 103–108.
Kalay S, Cakcak B, Oztekin O, Tezel G, Tosun O, Akcakus M, Oygur N . The role of VEGF and its soluble receptor VEGFR-1 in preterm newborns of preeclamptic mothers with RDS. J Matern Fetal Neonatal Med 2013; 26: 978–983.
Catarino C, Rebelo I, Belo L, Rocha S, Castro EB, Patrício B, Quintanilha A, Santos-Silva A . Fetal and maternal angiogenic/anti-angiogenic factors in normal and preeclamptic pregnancy. Growth Factors 2009; 27: 345–351.
Brownbill P, Mills TA, Soydemir DF, Sibley CP . Vasoactivity to and endogenous release of vascular endothelial growth factor in the in vitro perfused human placental lobule from pregnancies complicated by preeclampsia. Placenta 2008; 29: 950–955.
Tsao PN, Wei SC, Su YN, Chou HC, Chen CY, Hsieh WS . Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics 2005; 116: 468–472.
Salama RH, Fathalla MM, Mekki AR, Elsadek Bel-K . Implication of umbilical cord in preeclampsia. Med Princ Pract 2011; 20: 124–128.
Han SY, Jun JK, Lee CH, Park JS, Syn HC . Angiopoietin-2: a promising indicator for the occurrence of severe preeclampsia. Hypertens Pregnancy 2012; 31: 189–199.
Ross B, McIntosh M, Rodaros D, Hébert TE, Rohlicek CV . Systemic arterial pressure at maturity in rats following chronic hypoxia in early life. Am J Hypertens 2010; 23: 1228–1233.
Lamarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP . Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor {alpha} in pregnant rats. Hypertension 2008; 52: 1168–1172.
Anderson UD, Olsson MG, Kristensen KH, Åkerström B, Hansson SR . Review: biochemical markers to predict preeclampsia. Placenta 2012; 33 Suppl: S42–S47.
Kulkarni A, Mehendale S, Pisal H, Kilari A, Dangat K, Salunkhe S, Taralekar V, Joshi S . Association of omega-3 fatty acids and homocysteine concentrations in pre-eclampsia. Clin Nutr 2011; 30: 60–64.
Mehendale S, Kilari A, Dangat K, Taralekar V, Mahadik S, Joshi S . Fatty acids, antioxidants, and oxidative stress in pre-eclampsia. Int J Gynaecol Obstet 2008; 100: 234–238.
Sundrani DP, Reddy US, Joshi AA, Mehendale SS, Chavan-Gautam PM, Hardikar AA, Chandak GR, Joshi SR . Differential placental methylation and expression of VEGF, FLT-1 and KDR genes in human term and preterm preeclampsia. Clin Epigenetics 2013; 5: 6.
Acknowledgements
We thank the Department of Biotechnology (DBT), India, for funding the study. We also thank all the subjects who volunteered in this study and the nurses of Gupte Hospital who helped with sample collection. This study was funded by the Department of Biotechnology, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahay, A., Patil, V., Sundrani, D. et al. A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertens Res 37, 753–758 (2014). https://doi.org/10.1038/hr.2014.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.71
Keywords
This article is cited by
-
Hypoxia Inducible Factors (HIF1α and HIF3α) are differentially methylated in preeclampsia placentae and are associated with birth outcomes
Molecular and Cellular Biochemistry (2023)
-
Angiotensin II type 1 receptor agonistic autoantibody blockade improves postpartum hypertension and cardiac mitochondrial function in rat model of preeclampsia
Biology of Sex Differences (2021)
-
Unravelling the potential of angiogenic factors for the early prediction of preeclampsia
Hypertension Research (2021)
-
Lipoxin A4 suppresses angiotensin II type 1 receptor autoantibody in preeclampsia via modulating caspase-1
Cell Death & Disease (2020)
-
Placental hypoxia-induced alterations in vascular function, morphology, and endothelial barrier integrity
Hypertension Research (2020)