Abstract
To investigate the antihypertensive effects and tolerability of aliskiren in comparison with other antihypertensive drugs and placebo in patients with hypertension, a meta-analysis was performed of studies published between 1950 and 2012. A systematic literature search of MEDLINE and the Cochrane Library was conducted for randomized controlled trials. Weighted mean differences and relative risk with 95% confidence intervals were calculated for continuous and dichotomous data, respectively. In all, 14 studies with 6741 participants were included in the present meta-analysis. Nine studies included trial arms with placebo, four included angiotensin (Ang) AT1 receptor blockers (ARBs), three included Ang-converting enzyme inhibitors (ACEIs), two included calcium channel blockers (CCBs), one included a β-blocker, and one included hydrochlorothiazide (HCTZ). We found that aliskiren, which lowered blood pressure (BP) effectively in patients with mild-to-moderate hypertension, was similar to HCTZ but inferior to CCBs in BP reduction, response rates and control rates. Furthermore, aliskiren was superior to ACEIs in lowering diastolic BP (DBP), while it had similar effects to ACEIs on systolic BP (SBP) reduction, response rates and control rates. Additionally, the present meta-analysis showed the superiority of atenolol over aliskiren in DBP reduction and BP response but showed that atenolol was inferior in SBP reduction and BP control. No difference was found in the rates of therapeutic response between aliskiren and ARBs, while more patients achieved BP control with aliskiren. Further studies will be needed to determine the antihypertensive effects and tolerability of aliskiren in comparison with other antihypertensive drugs.
Similar content being viewed by others
Introduction
Hypertension is an important public health problem worldwide. The estimated total number of adults with hypertension in 2000 was 972 million (957–987 million): 333 million (329–336 million) in economically developed countries and 639 million (625–654 million) in economically developing countries.1 The number of adults with hypertension in 2025 is predicted to increase by ∼60%, to a total of 1.56 billion (1.54–1.58 billion).1 In addition to its high frequency, hypertension has been identified as the leading risk factor for cardiovascular and kidney diseases2, 3 and for mortality.4
The renin–angiotensin (Ang)–aldosterone system (RAAS) has a crucial role in volume regulation and the maintenance of blood pressure (BP), acting primarily through Ang II. Ang-converting enzyme inhibitors (ACEIs) and Ang AT1 receptor blockers (ARBs) reduce the effects of Ang II, either by reducing its production or by directly blocking its interaction with the Ang-1 receptor, respectively.5 Although ACEIs and ARBs have been proven effective, suppression of RAAS remains incomplete with these agents because of their disruption of the negative feedback effects of Ang II on renin release, with a consequent increase in plasma renin activity and the reactive activation of the RAAS.6 As renin catalyzes the conversion of angiotensinogen into Ang I, the initial and rate-limiting step of the RAAS, it has been suggested that renin inhibitors offer the potential to optimize suppression of the RAAS by interrupting the system at its first regulated step.7, 8
Aliskiren is the first orally effective direct renin inhibitor,9 and it has recently been approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of hypertension. It inhibits the activity of renin, controlling the renin system at the rate-limiting step. Some clinical trials have studied its efficacy in hypertension;10, 11 however, there have been no meta-analyses that have examined the effects of aliskiren and other antihypertensive agents on BP.
Therefore, we performed a meta-analysis to investigate the antihypertensive effects and tolerability of aliskiren, in comparison with other antihypertensive drugs and placebo, in patients with hypertension.
Materials and methods
Search strategy
We developed a protocol for the review and followed standard QUOROM reporting guidelines.12 We searched online databases, including MEDLINE (1950–2012) and the Cochrane Library (Issue 5, 2012), using the following terms: aliskiren, renin inhibitor, hypertension and BP, without restrictions on language. We also searched the Clinical Trials (http://clinicaltrials.gov/) Web site and the Novartis Clinical Trials Results Database (http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/main.jsp) for unpublished data and reviewed the reference lists of the included randomized controlled trials (RCTs) and review articles to identify other unrecognized or unpublished reports of RCTs.13, 14
Inclusion criteria
Studies were eligible for inclusion if they met the following criteria: (1) double-blind, prospective, randomized, controlled trials of antihypertensive treatment with aliskiren; (2) enrolled patients ⩾18 years of age with mild-to-moderate hypertension (systolic BP (SBP) 140–179 mm Hg and/or diastolic BP (DBP) 90–109 mm Hg, as defined in current international guidelines);15, 16 (3) study treatments had to have been taken for at least 4 weeks with aliskiren at 300 mg per day and other active comparators at the maximum commonly used dose; (4) only monotherapy treatment arms were included in the meta-analysis; and (5) assessment of the effectiveness of aliskiren vs. placebo or other classes of drugs for hypertension.
Exclusion criteria
The exclusion criteria pertained to studies that recruited participants with secondary hypertension, severe hypertension (DBP⩾110 mm Hg and/or SBP⩾180 mm Hg), poorly controlled diabetes mellitus, a history of severe cardiovascular or cerebrovascular disease, severe chronic kidney disease (stage 4–5), other severe life-threatening medical conditions, unavailable data or no quantitative data and that had cohorts of <25 patients. Studies that only enrolled patients in a specific subgroup (for example, based on ethnicity or diabetes mellitus) and that used aliskiren for indications other than hypertension were also excluded.
Data extraction
The quality of each included study was assessed on the Jadad quality scale,17 with a score of 0–2 reflecting low quality, a score of 3–4 indicating moderate quality and a score of 5 representing a high-quality study. All of the data were extracted by two independent authors. The following information was abstracted from each trial: number, age and sex of participants; intervention strategies; baseline SBP and DBP values; duration; and measures of end points (that is, changes from baseline in SBP and DBP, rates of therapeutic response and of SBP and DBP control; proportion of adverse events; and the number of drop-outs). Disagreements were resolved by discussion between the authors.
Statistical analysis
The meta-analysis was performed using the Cochrane RevMan software, version 5.02 (Cochrane Library, UK). For continuous data, the results were expressed as weighted mean differences (WMDs) with 95% confidence intervals (CIs). For dichotomous data, pooling data were described as relative risks (RRs) with 95% CIs. Subgroup analysis was conducted between different comparators. We assessed heterogeneity with a standard χ2-test, with significance set at P<0.10, and with the I2 statistic, with significance set at I2>50%.18 The fixed-effects model was employed in the absence of between study heterogeneity; otherwise, the random-effects model was used.
Results
Study characteristics
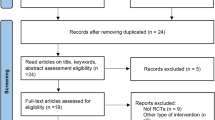
After screening the titles and abstracts of all of the studies identified by the search strategy, 491 potentially relevant articles were selected and reviewed for more detailed information. We excluded 30 duplicate articles, 35 non-related articles and 46 preclinical studies. Of the 380 reports selected for detailed evaluation, 359 studies were excluded, predominantly for study type (open-label, cross-over), study duration and article type (reviews, letters, comments, and interviews). Of the 21 full-text articles selected for assessing for eligibility, seven studies were not RCTs. Therefore, a total of 14 studies,10, 11, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 enrolling 6741 participants, fulfilled all of the inclusion criteria. All 14 articles were available as full reports (all in English). Table 1 describes the baseline characteristics of all of the eligible studies. The 14 studies included in this meta-analysis were short-term (4–16 weeks), randomized, double-blind clinical trials with similar designs and comparable primary end points and secondary efficacy measures. The patient demographics and baseline characteristics were also similar in all of the included studies, except that two studies that included patients aged older than 65 years old,27, 29 and one study included patients aged between 21 and 70 years old.26
Aliskiren was compared with ARBs (losartan, irbesartan and valsartan) in four trials,21, 23, 24, 26 ACEIs (ramipril, lisinopril) in two trials,10, 27 calcium channel blocker (CCB) (amlodipine) in two trials,19, 30 a β-blocker (atenolol) in one trial,20 a thiazide diuretic (hydrochlorothiazide, HCTZ)28 in one trial, and placebo in nine trials.11, 21, 22, 23, 24, 25, 28, 29, 30 Two reports of unpublished data were checked with the Novartis Clinical Trials Results Database and the Clinical Trials Web site, and they were designated CSPP100A240529 and CSPA100A2305.30 According to the Jadad quality scale, our quality assessment scores ranged from 3 to 5 for all of the studies included. These studies were considered to be of good quality, with six of them having excellent quality.
Outcome characteristics
Sitting clinic BP was used for all of the trials. The primary outcome of most of the trials was the change from baseline in mean sitting DBP, except for the studies by Verdecchia (2007)27 and by Stanton (2003),26 the primary outcomes of which were the change from baseline in mean 24 h ambulatory SBP, and for the study by Brown (2011)19 and for CSPP100A2405,29 the primary outcomes of which were the change from baseline in mean sitting SBP (msSBP). Secondary variables included the following: the change from baseline in msSBP, diastolic responder rates (defined as mean sitting DBP <90 mm Hg or equal to 10 mm Hg reduction in mean sitting DBP) or systolic responder rates (defined as msSBP <140 mm Hg or equal to 20 mm Hg reduction in msSBP); BP control rates (defined as a BP <140/90 mm Hg); number of patients with adverse events and withdrawals due to adverse effects.
Efficacy
Aliskiren vs. placebo
Nine trials11, 21, 22, 23, 24, 25, 28, 29, 30 involving 3541 patients compared the effects of treatments with aliskiren and placebo in terms of reduction in BP. Aliskiren was significantly superior to placebo in lowering DBP and SBP (WMD −4.75, 95% CI −5.59 to −3.92, P<0.00001; WMD −8.12, 95% CI −9.61 to −6.63, P<0.00001, respectively); Figures 1 and 2. Seven trials11, 23, 24, 25, 28, 29, 30 (n=2915) reported statistically significant improvements in responder rates (RR 1.65, 95% CI 1.52–1.80, P<0.00001); Figure 3. Six trials21, 23, 24, 25, 28, 30 (n=2577) reported that aliskiren led to significantly greater control rates than placebo (RR 1.96, 95% CI 1.73–2.23, P<0.00001); Figure 4.
Change (mm Hg) from baseline in mean sitting diastolic BP at end point following treatment with aliskiren or other antihypertensive drugs and placebo during monotherapy. ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CI, confidence interval; HCTZ, hydrochlorothiazide; IV, inverse variance. Control, antihypertensive drug used as comparator drug in that study. ‘Total’ indicates the total number of individuals.
Change (mm Hg) from baseline in mean sitting systolic BP at end point following treatment with aliskiren or other antihypertensive drugs and placebo during monotherapy. ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CI, confidence interval; HCTZ, hydrochlorothiazide; IV, inverse variance. Control, antihypertensive drug used as comparator drug in that study. ‘Total’ indicates the total number of individuals.
BP response rates of patients to aliskiren compared with other antihypertensive drugs and placebo. A successful response to treatment was defined as a mean sitting diastolic BP <90 mm Hg or a 10 ⩾ mm Hg reduction from baseline or a mean sitting systolic BP (msSBP) <140 mm Hg or a 20 ⩾ mm Hg reduction from baseline. ‘Events’ indicate number of patients with a successful response. ‘Total’ indicates the total number of individuals. ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BP, blood pressure; CI, confidence interval; HCTZ, hydrochlorothiazide; RR, relative risk; M-H, Mantek-Haenzel.
BP control rates of patients to aliskiren or other antihypertensive drugs and placebo. Control rate was defined as mean sitting diastolic BP <90 mm Hg and mean sitting systolic BP < 140 mm Hg. ‘Events’ indicate number of patients with a successful control. ‘Total’ indicates the total number of individuals. ACEIs. angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BP, blood pressure; CI, confidence interval; HCTZ, hydrochlorothiazide; RR, relative risk; M-H, Mantek-Haenzel.
Aliskiren vs. ARBs
Four trials21, 23, 24, 26 involving 1467 patients compared the effects of treatments with aliskiren and ARBs in terms of reduction in BP. No significant differences were found in the reduction of DBP and SBP of the two groups (WMD −0.83, 95% CI −2.79 to −1.13, P=0.40; WMD −0.57, 95% CI −2.40 to −1.25, P=0.54, respectively); Figures 1 and 2. Two trials23, 24 (n=1118) compared BP responder rates between aliskiren and ARBs, with no significant difference between the two groups (RR 0.99, 95% CI 0.89–1.10, P=0.84); see Figure 3. Three trials21, 23, 24 (n=1381) reported that more patients achieved BP control with aliskiren than with ARBs (RR 1.16, 95% CI 1.01–1.34, P=0.03); Figure 4. Additionally, Stanton (2003)26 showed that the reductions in daytime and nighttime ambulatory SBP and DBPs were of a similar magnitude with aliskiren and losartan.
Aliskiren vs. ACEIs
Two trials10, 27 involving 1022 patients compared the effects of treatments with aliskiren and ACEIs in terms of reduction in BP. Aliskiren significantly reduced DBP compared with ACEIs (WMD −1.49, 95% CI −2.49 to −0.49, P=0.003), while no difference was found in the reduction of SBP of the two groups (WMD −1.76, 95% CI −4.55 to −1.04, P=0.22); Figures 1 and 2. Only one trial10 (n=832) compared BP responder rates between aliskiren and ACEIs, with no significant difference between the two groups (RR 1.10, 95% CI 0.99–1.24, P=0.09); Figure 3. There was no significant difference in BP control rates between the two groups (RR 1.12, 95% CI 0.96–1.30, P=0.15); Figure 4. In addition, Verdecchia27 found that both aliskiren and lisinopril groups produced significant decreases in mean 24 h ambulatory SBP and ambulatory DBP from baseline to end point.
Aliskiren vs. amlodipine
Only one study30 involving 384 patients reported the effects of treatments with aliskiren and amlodipine in terms of reduction in BP. Aliskiren was inferior to amlodipine in lowering DBP and SBP (WMD 3.63, 95% CI 1.85–5.41, P<0.0001; WMD 5.67, 95% CI 2.86–8.48, P<0.0001, respectively); Figures 1 and 2. Two trials19, 30 (n=1005) reported the response rates. Response rates were significantly higher with amlodipine than with aliskiren (RR 0.77, 95% CI 0.69–0.86, P<0.00001); Figure 3. Only one trial30 (n=380) reported the control rates. The control rates were significantly higher with amlodipine than with aliskiren (RR 0.72, 95% CI 0.57–0.91, P=0.006); Figure 4.
Aliskiren vs. HCTZ
Only one study28 involving 359 patients reported the effects of treatments with aliskiren and HCTZ in terms of reduction in BP. No difference was found in the reduction of DBP and SBP of the two groups (WMD −0.90, 95% CI −2.56–0.76, P=0.29; WMD −1.40, 95% CI −4.04–1.24, P=0.30, respectively); Figures 1 and 2. The trial28 (n=353) compared BP responder rates between aliskiren and HCTZ, with no significant difference between the two groups (RR 1.08, 95% CI 0.92–1.28, P=0.34); Figure 3. There was no significant difference in BP control rates between the two groups (RR 1.24, 95% CI 0.97–1.59, P=0.09); Figure 4.
Aliskiren vs. atenolol
Only one study20 involving 462 patients reported the effects of treatments with aliskiren and atenolol in terms of reduction in BP. DBP changes were larger with atenolol than with aliskiren (WMD 2.40, 95% CI 0.74–4.06, P=0.004), while there was no significant difference between the msSBP reductions of the two groups (WMD −0.08, 95% CI −3.02–2.86, P=0.96); Figures 1 and 2. Only one trial20 (n=460) reported response rates. Response rates were significantly higher with atenolol than aliskiren (RR 0.84, 95% CI 0.74–0.95, P=0.007); Figure 3. There was no significant difference in BP control rates between the two groups (RR 0.86, 95% CI 0.68–1.08, P=0.18) of the trial;20 Figure 4.
Safety and tolerability
The safety population comprised all of the patients randomized in the 14 trials included in this analysis (n=7879). The most common adverse events included headache, dizziness, diarrhea, nasopharyngitis, fatigue and nausea. There were no significant differences in number of adverse events between aliskiren and placebo or other active comparators (Table 2). Similarly, there were no significant differences in withdrawals due to adverse events between aliskiren and placebo or other active comparators (Table 2).
Discussion
The present study evaluated the antihypertensive efficacy, safety, and tolerability of the novel, orally effective direct renin inhibitor aliskiren in comparison with other antihypertensive agents, in a total of 6741 patients with mild-to-moderate hypertension.
The results of our study suggest that aliskiren, which lowered BP effectively in patients with mild-to-moderate hypertension, was similar to HCTZ but inferior to CCBs in BP reduction, response rates and control rates. Furthermore, aliskiren was superior to ACEIs in lowering DBP, while it had similar effects to ACEIs on SBP reduction, response rates and control rates. Additionally, the present meta-analysis showed the superiority of atenolol over aliskiren in DBP reduction and BP response and atenolol’s inferiority in SBP reduction and BP control. No difference was found in rates of therapeutic response between aliskiren and ARBs; these findings are consistent with the results from two previous studies,31, 32 which demonstrated that aliskiren and ARBs have comparable efficacy, while we found that more patients achieved BP control with aliskiren. The most obvious difference between the two studies31, 32 and ours is that they only assessed the antihypertensive efficacy and safety of aliskiren in comparison with ARBs in mild-to-moderate hypertensive patients, while our study included other antihypertensive drugs, apart from ARBs, for analysis. The two other meta-analyses31, 32 included study treatments with aliskiren at doses of 150 and 300 mg, drug combinations, and specific groups, such as obese patients. Zheng et al.31 found that aliskiren monotherapy at 150 mg per day provided comparable antihypertensive efficacy to ARBs at half of the recommended maximum dose; moreover, aliskiren and ARB combination therapy provided more effective BP reduction than each respective monotherapy, without increasing adverse events, and this combination might have organ-protective effects.
Aliskiren provides highly effective BP reduction independent of age or sex in patients with hypertension. In a pooled analysis by Weir,33 aliskiren demonstrated comparable efficacy in patients aged ⩾65 years old or<65 years old, in men and women, and it lowered BP effectively in all racial subgroups, consistent with previous studies.34, 35
In addition to its antihypertensive effects, some recent publications have published findings regarding aliskiren’s protective profile on the organs. Aliskiren has been shown to induce reductions in plasma levels of B-type natriuretic peptide in heart failure36 and in left ventricular mass in hypertensive patients with left ventricular hypertrophy.37 In addition, the AVOID trial showed that aliskiren has renoprotective effects that are independent of its BP lowering effects in hypertensive type 2 diabetic patients with nephropathy who are receiving the recommended maximal renoprotective treatment.38
Our meta-analysis indicates that aliskiren is associated with a similar incidence of adverse events and discontinuation due to adverse events to placebo and that it has similar tolerability with other antihypertensive drug classes. A pooled analysis of >12 000 patients confirmed our results, which demonstrate that aliskiren at doses of 150 mg or 300 mg exhibits an excellent safety profile in patients with hypertension.39 These findings suggest that aliskiren could be widely used for the treatment of hypertension because tolerability is an important factor in patient non-compliance and quality of life.40
We analyzed the short-term antihypertensive effects and tolerability of aliskiren in patients with mild-to-moderate hypertension. The longest-duration randomized, controlled trial, Brown (2011),19 was 32 weeks, while the most common trial length was eight weeks. Nevertheless, White’s Pooled Analysis found that more than 750 patient-years of aliskiren exposure confirmed the tolerability of aliskiren in long-term studies.39 A pooled analysis reported that rates of discontinuation due to adverse events were low (1.7%–2.6%); the most frequently reported adverse events with aliskiren were headache (5.7%), nasopharyngitis (4.4%), diarrhea (2.6%), dizziness (1.8%) and fatigue (1.6%).33
A meta-analysis of the result of serious adverse events was not conducted because of the lack of effective data in Villamil28 and in Pool.24 However, the incidence of serious adverse events was very low: three trials10, 23, 26 reported one case of death in each in the losartan,26 aliskiren,23 and valsartan23 and ramipril group.10 Although aliskiren has shown a good safety and tolerability profile, we should note that the Food and Drug Administration has recently issued a warning about combining aliskiren with ACEIs and ARBs in patients diagnosed with diabetes or renal impairment. The ALTITUDE study, which was recently terminated, found an increased risk of adverse events in patients considered high-risk and who were taking direct renin inhibitors as an add-on to other antihypertensive medications, such as ARBs and ACEIs. The Food and Drug Administration said in a safety announcement that diabetic patients who combine the drugs are at risk of renal impairment, hypotension and hyperkalemia, although the combination showed similar safety and tolerability in patients with uncomplicated hypertension.
Change from baseline in mean sitting DB was the primary outcome in most of the trials included in our analysis, while two trials27, 29 with population aged older than 65 years used change from baseline in SBP as the primary outcome. Usually, diastolic hypertension predominates before age 50, and systolic hypertension represents the most common form of hypertension in patients older than 50 years of age;41 therefore, SBP is the main efficacy variable for the diagnosis and treatment of hypertension in the elderly.42
To the best of our knowledge, this is the only systematic review summarizing the antihypertensive effects and tolerability of aliskiren in comparison with other antihypertensive drugs. There are still some limitations to our meta-analysis. First, each result of our meta-analysis was based only on limited trials with limited sizes; in particular, there was only one single study comparing aliskiren with atenolol and one comparing HCTZ and amlodipine. The results should be interpreted cautiously, and more RCTs are necessary to support our findings. Actually, there are some ongoing trials with large-scale populations that will provide further data on the efficacy and tolerability of aliskiren.43, 44, 45 Second, the duration of the trials in our analysis was relatively short. Third, we dealt with surrogate end points, instead of direct clinical outcomes, such as the incidence of cardiovascular disease or morbidity and mortality.
Conclusion
Our meta-analysis indicates that the direct renin inhibitor aliskiren, at doses of 300 mg, provided good antihypertensive efficacy that was at least as effective as that provided by ACEIs, ARBs and HCTZ at the recommended daily doses. However, aliskiren might not be as effective as CCBs and β-blockers, although it has similar safety and tolerability in patients with hypertension. Additional large-scale, well-controlled trials, preferably with clinical end points, are warranted.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223.
Poulter N . Global risk of cardiovascular disease. Heart 2003; 89 (Suppl 2): ii2–ii5 discussion ii35–37.
Lea JP, Nicholas SB . Diabetes mellitus and hypertension: key risk factors for kidney disease. J Natl Med Assoc 2002; 94: 7S–15S.
Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ . The Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–1360.
Watanabe T, Barker TA, Berk BC . Angiotensin II and the endothelium: diverse signals and effects. Hypertension 2005; 45: 163–169.
Weber MA, Giles TD . Inhibiting the renin-angiotensin system to prevent cardiovascular diseases: do we need a more comprehensive strategy? Rev Cardiovasc Med 2006; 7: 45–54.
Skeggs LT, Kahn JR, Lentz K, Shumway NP . The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med 1957; 106: 439–453.
Duprez DA . Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens 2006; 24: 983–991.
Wood JM, Maibaum J, Rahuel J, Grütter MG, Cohen NC, Rasetti V, Rüger H, Göschke R, Stutz S, Fuhrer W, Schilling W, Rigollier P, Yamaguchi Y, Cumin F, Baum HP, Schnell CR, Herold P, Mah R, Jensen C, O'Brien E, Stanton A, Bedigian MP . Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun 2003; 308: 698–705.
Andersen K, Weinberger MH, Egan B, Constance CM, Ali MA, Jin J, Keefe DL . Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6-month, randomized, double-blind trial. J Hypertens 2008; 26: 589–599.
Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL . Aliskiren a novel oral renin inhibitor, provides dose-dependent efficacy and placebo-like tolerability in Japanese patients with hypertension. Hypertens Res 2006; 29: 997–1005.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF . Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999; 354: 1896–1900.
Hopewell S, McDonald S, Clarke M, Egger M . Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev 2007; 2: MR000010.
McAuley L, Pham B, Tugwell P, Moher D . Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000; 356: 1228–1231.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B . Management of arterial hypertension of the European Society of Hypertension, European Society of Cardiology. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187.
World Health Organization. International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–1992.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12.
Higgins JP, Thompson SG, Deeks JJ, Altman DG . Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560.
Brown MJ, McInnes GT, Papst CC, Zhang J, MacDonald TM . Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet 2011; 377: 312–320.
Dietz R, Dechend R, Yu CM, Bheda M, Ford J, Prescott MF, Keefe DL . Effects of the direct renin inhibitor aliskiren and atenolol alone or in combination in patients with hypertension. J Renin Angiotensin Aldosterone Syst 2008; 9: 163–175.
Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP . Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005; 111: 1012–1018.
Oh BH, Mitchell J, Herron JR, Chung J, Khan M, Keefe DL . Aliskiren an oral renin inhibitor, provides dose-dependent efficacy and sustained 24-hour blood pressure control in patients with hypertension. J Am Coll Cardiol 2007; 49: 1157–1163.
Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A . Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370: 221–229.
Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, Chiang Y, Satlin A . Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens 2007; 20: 11–20.
Puig JG, Schunkert H, Taylor AA, Boye S, Jin J, Keefe DL . Evaluation of the dose–response relationship of aliskiren, a direct renin inhibitor, in an 8-week, multicenter, randomized, double-blind, parallel-group, placebo-controlled study in adult patients with stage 1 or 2 essential hypertension. Clin Ther 2009; 31: 2839–2850.
Stanton A, Jensen C, Nussberger J, O'Brien E . Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension 2003; 42: 1137–1143.
Verdecchia P, Calvo C, Möckel V, Keeling L, Satlin A . Safety and efficacy of the oral direct renin inhibitor aliskiren in elderly patients with hypertension. Blood Press 2007; 16: 381–391.
Villamil A, Chrysant SG, Calhoun D, Schober B, Hsu H, Matrisciano-Dimichino L, Zhang J . Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25: 217.
An eight-week double-blind, multi-center, randomized, placebo-controlled, parallel-group study to evaluate the efficacy and safety of aliskiren 75 mg, 150 mg and 300 mg in elderly patients with essential hypertension when given with a light meal. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=2933.
An 8-week double-blind, multicenter, randomized, multifactorial, placebo-controlled, parallelgroup study to evaluate the efficacy and safety of aliskiren administered alone and in combination with amlodipine in patients with essential hypertension. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do?trialResult=3000.
Zheng Z, Shi H, Jia J, Li D, Lin S . A systematic review and meta-analysis of aliskiren and angiotension receptor blockers in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst 2011; 12: 102–112.
Gao D, Ning N, Niu X, Wei J, Sun P, Hao G . Aliskiren vs. angiotensin receptor blockers in hypertension: meta-analysis of randomized controlled trials. Am J Hypertens 2011; 24: 613–621.
Weir MR, Bush C, Anderson DR, Zhang J, Keefe D, Satlin A . Antihypertensive efficacy, safety, and tolerability of the oral direct renin inhibitor aliskiren in patients with hypertension: a pooled analysis. J Am Soc Hypertens 2007; 1: 264–277.
Gradman AH, Weir MR, Wright M, Bush CA, Keefe DL . Efficacy, safety and tolerability of aliskiren, a direct renin inhibitor, in women with hypertension: a pooled analysis of eight studies. J Hum Hypertens 2010; 24: 721–729.
Dahlöf B, Anderson D, Arora V, Bush C, Keefe D . Aliskiren a direct renin inhibitor, provides antihypertensive efficacy and excellent tolerability independent of age or gender in patients with hypertension. J Clin Hypertens 2007; 9: A157.
McMurray JJV, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, Ford J, Verma A, Lewsey J ; Aliskiren Observation of Heart Failure Treatment (ALOFT) Investigators. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail 2008; 1: 17–24.
Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, Papst CC, Smith BA, Dahlöf B . Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation 2009; 119: 530–537.
Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK . AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433–2446.
White WB, Bresalier R, Kaplan AP, Palmer BF, Riddell RH, Lesogor A, Chang W, Keefe DL . Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens 2010; 12: 765–775.
Düsing R, Weisser B, Mengden T, Vetter H . Changes in antihypertensive therapy--the role of adverse effects and compliance. Blood Press 1998; 7: 313–315.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ . Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252.
Dickerson LM, Gibson MV . Management of hypertension in older persons. Am Fam Physician 2005; 71: 469–476.
Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Böhm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A, ; on behalf of the ASTRONAUT investigators and study coordinators. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT). Eur J Heart Fail 2011; 13: 100–106.
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z, Armbrecht J, Pfeffer MA . Aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant 2009; 24: 1663–1671.
Krum H, Massie B, Abraham WT, Dickstein K, Kober L, McMurray JJ, Desai A, Gimpelewicz C, Kandra A, Reimund B, Rattunde H, Armbrecht J . Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur J Heart Fail 2011; 13: 107–114.
Acknowledgements
We thank all of the participants in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, Y., Meng, L., Shao, H. et al. Aliskiren vs. other antihypertensive drugs in the treatment of hypertension: a meta-analysis. Hypertens Res 36, 252–261 (2013). https://doi.org/10.1038/hr.2012.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.185
Keywords
This article is cited by
-
Clinical efficacy, safety and tolerability of Aliskiren Monotherapy (AM): an umbrella review of systematic reviews
BMC Cardiovascular Disorders (2020)
-
The role of aliskiren in the management of hypertension and major cardiovascular outcomes: a systematic review and meta-analysis
Journal of Human Hypertension (2019)
-
Combined Aliskiren and L-arginine treatment reverses renovascular hypertension in an animal model
Hypertension Research (2015)
-
Combining drugs to optimize the therapy of hypertension: experimental evidence derived from animal models
Hypertension Research (2015)
-
Role of the Renin-Angiotensin-Aldosterone System and Its Pharmacological Inhibitors in Cardiovascular Diseases: Complex and Critical Issues
High Blood Pressure & Cardiovascular Prevention (2015)