Abstract
Hypertension is associated with the imbalance of vasoconstrictor and vasodilator systems. Vasodilation is usually evaluated in isolated blood vessels, but except for nitric oxide (NO), relatively little attention is given to the in vivo efficiency of particular vasodilator mechanisms. The aim of our study was to evaluate the contribution of endogenous vasodilator prostanoids, Ca2+-activated K+ channels and NO to blood pressure (BP) maintenance in rats with three different forms of experimental hypertension. Both principal vasopressor systems (the renin–angiotensin system and the sympathetic nervous system) were blocked by captopril and pentolinium in conscious spontaneously hypertensive rats (SHRs), Dahl salt-hypertensive (DS-HS) rats and rats with NO-deficient hypertension, as well as in their normotensive controls. Thereafter, we monitored BP changes in rats subjected to either a sequential or an isolated blockade of prostanoid synthesis by the non-selective cyclooxygenase inhibitor, indomethacin, of Ca2+-activated K+ channels by tetraethylammonium and of NO formation by NG-nitro-L-arginine methyl ester. All three forms of experimental hypertension were characterized by augmented sympathetic vasoconstriction. The vasodilatation exerted by endogenous prostanoids and Ca2+-activated K+ channels was enhanced in all forms of hypertension, almost proportionally to BP elevation. On the contrary, NO-dependent vasodilatation was not enhanced in any form of experimental hypertension, and there was a severe relative NO deficiency in both, SHRs and DS-HS rats. In conclusion, our data suggested that there is a compensatory activation of vasodilator prostanoids and Ca2+-activated K+ channels in rats with experimental hypertension, whereas NO-dependent vasodilatation is not augmented. Thus, the overall activity of vasodilator systems failed to compensate for augmented sympathetic vasoconstriction in hypertensive animals.
Similar content being viewed by others
Introduction
Hypertension is often associated with the imbalance between vasoconstrictor and vasodilator mechanisms. Considerable attention has been paid to the in vivo alterations of various pressor systems (for example, angiotensin II, norepinephrine, endothelin-1 and vasopressin) in different forms of experimental hypertension. On the contrary, the efficiency of vasodilator systems is usually evaluated in isolated blood vessels, in which the acetylcholine-induced relaxation of arteries precontracted with catecholamines is studied to reveal the so-called endothelial dysfunction. This evaluation is often performed in large conduit arteries but not in small resistance arteries, which are responsible for the control of peripheral resistance and blood pressure (BP). Although the estimation of the contribution of particular vasoactive systems to BP maintenance in conscious rats might be rather difficult, this approach yields valuable data that enable us to evaluate the altered efficiency of particular vasoconstrictor and vasodilator systems in hypertension.
In the last decade, our research effort has been focused on the balance between sympathetic vasoconstriction and nitric oxide (NO)-dependent vasodilatation in salt,1 NO-deficient2 and genetic hypertension.3, 4 All these forms of experimental hypertension are characterized by sympathetic hyperactivity leading to an enhanced Ca2+ influx through L-type voltage-dependent Ca2+ channels (L-VDCCs), which is reflected by an enhanced BP response to the acute administration of Ca2+ channel blockers, such as nifedipine.4, 5
Much less attention has been paid to vasodilator systems (except for NO) in various forms of experimental hypertension. It seems that the magnitude of the BP response to acute inhibition of NO synthase by NG-nitro-L-arginine methyl ester (L-NAME) injection is preserved in both salt and spontaneous hypertension,1, 4 but NO-dependent vasodilatation is clearly insufficient in compensating for major sympathetic hyperactivity, indicating a relative NO deficiency in rats with these forms of hypertension.6, 7
The synthesis and/or release of vasodilator prostanoids (namely prostacyclin (PGI2)) has been found to be increased in the blood vessels of Dahl salt-hypertensive (DS-HS) rats,8, 9 DOCA-salt hypertensive rats and spontaneously hypertensive rats (SHRs),10, 11 but not in prehypertensive SHRs.11, 12 Few attempts have been made to evaluate the contribution of these prostanoids to BP regulation. Acute indomethacin infusion did not influence the BP of either normotensive rats or SHRs, but it considerably augmented their BP response to the inhibition of NO synthase by L-NAME.13, 14 Moreover, an acute administration of indomethacin or meclofenamate (non-selective cyclooxygenase (COX) inhibitors) increased the vascular resistance in isolated perfused hindquarters of stroke-prone SHRs, but it did not augment the vascular response to adrenergic stimulation.15, 16 In addition, chronic meclofenamate administration was reported to augment the salt-induced BP rise and the renal vascular resistance in SHRs.17, 18 Chronic meclofenamate infusion also increased BP in normotensive rats when they were subjected to a chronic infusion of subpressor doses of L-NAME.19 These observations suggest a possible participation of vasodilator prostanoids in BP control, at least in some hypertensive models.
Another highly interesting vasodilator mechanism is based upon Ca2+-activated K+ channels; the activation of which leads to membrane hyperpolarization and a reduction of Ca2+ influx through L-VDCCs.20, 21 Increased Ca2+-dependent K+ turnover was reported in conduit arteries of SHRs22 and DOCA-salt hypertensive rats23 almost 40 years ago. The importance of this system for BP control and spontaneous hypertension development has been demonstrated by Furspan and Bohr.24, 25 Unfortunately, little has been done to evaluate the role of this system in the in vivo BP control, although the inhibitors of both large conductance Ca2+-activated K+ channels (BKCa) and small or intermediate Ca2+-activated K+ channels (SKCa, IKCa) are available (for review see Ledoux et al.21). For example, tetraethylammonium (TEA, in concentrations below 1 mmol l−1) can be used for the inhibition of BKCa under the in vivo conditions.
In this study, we attempted to evaluate the efficiency of the three above-mentioned vasodilator systems (prostanoids, BKCa and NO) in three different models of experimental hypertension—genetic hypertension (SHRs), NO-deficient hypertension (L-NAME treated rats) and salt-induced hypertension (Dahl rats). Using captopril- and pentolinium-pretreated rats to eliminate the principal pressor systems (the renin–angiotensin system and the sympathetic nervous system), we performed either a sequential blockade of all three vasodilator systems or an isolated blockade of each system to avoid the influence of a previous blockade of other vasodilator system(s). The aim of our study was to test the hypothesis on the relative (but not absolute) deficiency of vasodilator mechanisms in experimental hypertension. We also tested whether any of these mechanisms show signs of compensatory enhancement to counterbalance the existing sympathetic hyperactivity.
Methods
Animals
Studies were performed on three different models of experimental hypertension: genetic hypertension, NO-deficient hypertension and salt-induced hypertension. Two to four animals were housed per cage under controlled conditions (temperature 23±1 °C, 12-h light/dark cycles) with ad libitum access to tap water and standard ST-1 rat chow (different types of diet were used for salt-induced hypertension; see below). All procedures and experimental protocols were approved by the Ethical Committee of the Institute of Physiology AS CR and conformed to the European Convention on Animal Protection and Guidelines on Research Animal Use.
Genetic hypertension
The experiments were performed in 20-week-old male SHRs and normotensive Wistar–Kyoto rats (WKY) from the colony of the Institute of Physiology AS CR, Prague, established from breeding pairs purchased at Charles River.
NO-deficient hypertension
At the age of 12 weeks, male Wistar rats (from the colony of the Institute of Physiology AS CR, Prague) were randomly divided into control (WIS) or experimental groups (LN). Animals in the LN group were treated with the NO synthase inhibitor L-NAME (40 mg kg−1 of body weight per day, administered in the drinking water) for 5 weeks.
Salt-induced hypertension
The experiments were performed in the female animals of two inbred strains—Dahl salt-sensitive SS/Jr (DS) and salt-resistant SR/Jr (DR) rats from the colony of the Institute of Physiology AS CR, Prague (initial breeding pairs were kindly provided by Professor John P Rapp). After weaning at the age of 4 weeks, animals of both strains were fed either a low-salt (LS, 0.3% NaCl) or a high-salt (HS, 5% NaCl) diet for 6 weeks.
Blood pressure measurement
One day before BP determination, polyethylene catheters were inserted into the left carotid artery (PE 50) and the jugular vein (PE 10) under 2% isoflurane anesthesia and exteriorized in the interscapular region. The BP was recorded in conscious rats after a 24-h recovery using the PowerLab system (ADInstruments, Bella Vista, Australia). Four animals (chosen in random order) were always recorded simultaneously. To eliminate the influence of circadian BP variation, the measurements were always performed between 0800 and 1130 hours.
Protocol 1: Sequential blockade of three vasodilator systems
The scheme of Protocol 1 is shown in Figure 1a. The baseline BP values were monitored in conscious animals for 30 min (basal values). Thereafter, a sequential blockade of the principal pressor systems, the renin–angiotensin system and the sympathetic nervous system, was performed according to a modified protocol of Minami et al.26 as described by Zicha et al.1 Initially, an i.v. bolus of captopril (angiotensin converting enzyme inhibitor, 10 mg kg−1 body weight) was injected to block angiotensin-dependent vasoconstriction. After 10 min, sympathetic vasoconstriction was eliminated by a ganglionic blockade induced by pentolinium (5 mg kg−1 body weight). When the decreased BP was temporarily stabilized for approximately 5 min at minimum values, a successive blockade of the depressor systems (formation of prostanoids, opening of BKCa channels and NO synthesis) was performed. This sequence of particular system blockades was chosen on the basis of our preliminary experiments (Behuliak and Zicha, unpublished data), indicating that a blockade of prostanoids or BKCa channels after the inhibition of NO synthase yielded only small BP changes. First, the i.v. administration of the non-selective COX inhibitor indomethacin (10 mg kg−1 body weight followed by infusion at a rate of 1 mg kg−1 min−1) lowered the formation of prostanoids. After the stabilization of BP elevation (10 min later), an i.v. bolus of TEA (20 mg kg−1 body weight) was injected to prevent the opening of BKCa channels. Finally, after the disappearance of the transient TEA-induced BP peak, the NO synthase inhibitor L-NAME (30 mg kg−1 body weight) was administered, and the BP elevation was monitored for the next 20 min.
(a) Protocol 1: Representative authentic recordings of blood pressure changes in conscious rats after the sequential i.v. blockade of pressor systems, renin–angiotensin system (RAS, 10 mg kg−1 body weight i.v. captopril) and the sympathetic nervous system (SNS, 5 mg kg−1 body weight i.v. pentolinium), followed by successive blockade of depressor systems—blockade of prostanoids formation by a non-selective cyclooxygenase inhibitor (10 mg kg−1 indomethacin followed by its infusion 1 mg kg−1 min−1 body weight i.v.), prevention of the opening of BKCa channels by tetraethylammonium (20 mg kg−1 body weight i.v. tetraethylammonium (TEA)) and inhibition of nitric oxide (NO) synthase by NG-nitro-L-arginine methyl ester (L-NAME, 30 mg kg−1 body weight i.v.). (b) In Protocol 2, the blockade of pressor systems (RAS and SNS) was followed by the blockade of NO synthesis (30 mg kg−1 body weight i.v. L-NAME), whereas the blockade of BKCa channels (c) was studied in Protocol 3 (20 mg kg−1 body weight i.v. TEA). These protocols were same for all the three models of experimental hypertension.
Protocols 2 and 3: Isolated blockades of particular vasodilator systems
Baseline BP values and the sequential blockade of pressor systems (renin–angiotensin system by captopril, and sympathetic nervous system by pentolinium) were monitored and performed in the same way as in Protocol 1 (Figure 1a). After the temporary BP stabilization for approximately 5 min following pentolinium administration, an isolated blockade of NO synthesis (L-NAME, 30 mg kg−1 body weight) was performed in Protocol 2 (Figure 1b). In Protocol 3, pentolinium administration was followed by TEA injection, which prevented the opening of BKCa channels (Figure 1c). Data for the isolated blockade of prostanoid formation (non-selective COX inhibition by indomethacin) were analyzed from Protocol 1 (Figure 1a).
Drugs
All drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA). Indomethacin was dissolved in 160 mM Na2CO3, and all the other drugs were dissolved in a saline solution (0.9% NaCl). The drugs were injected as an i.v. bolus in a volume of 1 ml kg−1 body weight by a catheter inserted into the jugular vein. For each experiment, we prepared fresh drug solutions. Additional rats (n=3) were injected with Na2CO3 solution as a vehicle control group. This small quantity of Na2CO3 did not produce any BP changes in the intact animals or the rats pretreated with captopril and pentolinium.
Data analysis and statistics
All data are presented as the mean±s.e.m. The effects of the drugs were analyzed as absolute changes (ΔBP) and relative BP changes (% ΔBP; expressed as a percentage of the basal values of BP). For a two-group comparison, Student's t-test was performed. For a multiple group analysis, one-way analysis of variance and post-hoc Fisher's least significant difference were used. P<0.05 was considered statistically significant.
Results
All the three forms of experimental hypertension were characterized by substantial elevation in the basal mean arterial pressure (MAP, Figure 2) due to a pronounced increase in the sympathetic BP component, which was estimated on the basis of pentolinium-induced MAP reduction (Figures 3a–c). The enhancement of sympathetic vasoconstriction was proportional to the BP elevation in all hypertensive models because there was no significant difference between the hypertensive rats and their normotensive controls, if the pentolinium-sensitive MAP component was expressed as a percentage of the basal MAP (Figures 3d–f). However, the direct pressor contribution of angiotensin II (estimated on the basis of captopril-induced MAP reduction) to BP maintenance was much smaller (below 10 mm Hg) in all of the studied forms of experimental hypertension, and there was no significant difference between the hypertensive and the normotensive animals (data not shown).
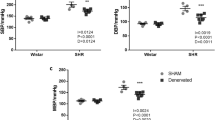
Basal values of mean arterial pressure (MAP) in three models of experimental hypertension (Protocol 1). (a) Genetic hypertension: spontaneously hypertensive rats (SHRs, n=11) and normotensive Wistar–Kyoto rats (WKY, n=6); *P<0.05 from WKY rats. (b) Nitric oxide-deficient hypertension: NG-nitro-L-arginine methyl ester treated rats (LN, n=6) and normotensive Wistar rats (WIS, n=11); *P<0.05 from WIS. (c) Salt-induced hypertension: Dahl salt-sensitive (DS) and salt-resistant (DR) rats fed with high-salt (DS-HS and DR-HS) or low-salt diet (DS-LS and DR-LS; n=8 in each group); *P<0.05 from DS-LS; #P<0.05 from DR-HS; +P<0.05 from DR-LS rats. Data are expressed as the mean±s.e.m.
The absolute (a–c) and relative (d–f) changes of mean arterial pressure (MAP) induced by blockade of the sympathetic nervous system by pentolinium (5 mg kg−1 body weight i.v.) in captopril-pretreated rats (Protocol 1). Relative values (d–f) represent MAP changes expressed as a percentage of basal MAP. Data are expressed as the mean±s.e.m. For other legends, see Figure 2.
The infusion of indomethacin caused a pronounced lasting BP elevation in the rats pretreated with captopril and pentolinium (Figure 1a). This effect was always significantly greater in the hypertensive rats compared with their respective controls (Figures 4a–c). The difference in the vasodilator contribution of prostanoids was greatest in SHRs (Figure 4a), but the relative vasodilator efficiency of this vasodilator system was not significantly altered in any hypertensive models (Figures 4d–f).
The absolute (a–c) and relative (d–f) changes in mean arterial pressure (MAP) induced by a non-selective cyclooxygenase inhibitor (10 mg kg−1 indomethacin followed by its infusion, 1 mg kg−1 min−1 body weight i.v.) in captopril- and pentolinium-pretreated rats (Protocol 1). Data are expressed as the mean±s.e.m. For other legends, see Figure 2.
The acute blockade of BKCa channels by TEA injection caused a considerable, but transient, BP peak (Figures 1a and c), which was again more pronounced in all three forms of hypertension (Figures 5a–c). However, the relative efficiency of these K+ channels in modifying the BP was never increased in the rats with either form of experimental hypertension (Figures 5d–f).
The absolute (a–c) and relative (d–f) changes of mean arterial pressure (MAP) induced by the transient blockade of BKCa channels (20 mg kg−1 body weight i.v. tetraethylammonium) in captopril- and pentolinium-pretreated rats after the blockade of prostanoids formation by indomethacin (Protocol 1). Data are expressed as the mean±s.e.m. For other legends, see Figure 2.
On the contrary, BP changes induced by the acute inhibition of NO synthase indicated a similar L-NAME induced BP rise in the SHRs and WKY rats (Figure 6a). Of course, the BP response was greatly attenuated in the NO-deficient rats (Figure 6b). There was a moderate reduction in the BP response in the DS-HS rats compared with normotensive DS-LS rats, but the BP response to acute L-NAME in hypertensive DS-HS animals did not differ from that of normotensive DR-HS animals (Figure 6c). When L-NAME induced BP changes were expressed in terms of basal MAP, it was evident that the relative vasodilator efficiency of the NO system was considerably attenuated, not only in NO-deficient rats but also in the SHRs and DS-HS rats (Figures 6d–f). These findings indicate the important alterations of this vasodilator system in all three forms of experimental hypertension.
The absolute (a–c) and relative (d–f) changes in mean arterial pressure (MAP) induced by nitric oxide synthase inhibitor (30 mg kg−1 body weight i.v. NG-nitro-L-arginine methyl ester) in captopril- and pentolinium-pretreated rats after the blockade of prostanoids formation by indomethacin and the transient blockade of BKCa channels by tetraethylammonium (Protocol 1). Data are expressed as the mean±s.e.m. For other legends, see Figure 2.
When all the rats studied using Protocol 1 were combined (irrespective of the form of experimental hypertension), we could evaluate the contribution of the particular vasoconstrictor and vasodilator systems to BP maintenance. It is evident that the basal MAP level was proportional to sympathetic vasoconstriction (pentolinium-induced MAP changes; Figure 7a). The compensatory activation of vasodilator prostanoids and Ca2+-activated K+ channels was indicated by the highly significant correlations between basal MAP and either indomethacin-induced or TEA-induced MAP changes (Figures 7b and c). On the contrary, there was no significant relationship between L-NAME induced MAP changes and the basal MAP level (Figure 7d).
Relationships between mean arterial pressure (MAP) and absolute BP changes induced by (a) blockade of sympathetic nervous system (pentolinium); (b) blockade of prostanoids formation by non-selective cyclooxygenase inhibitor (indomethacin); (c) transient blockade of BKCa channels (tetraethylammonium, TEA); and (d) blockade of nitric oxide (NO) synthase (NG-nitro-L-arginine methyl ester, L-NAME). Triangles represent genetic hypertension (spontaneously hypertensive rats and Wistar–Kyoto rats), squares salt-induced hypertension (Dahl rats) and circle NO-deficient hypertension (LN and WIS rats).
In further experiments, we studied the effects of an isolated blockade of either NO synthesis or BKCa channels using additional subgroups of rats with the three above-mentioned forms of experimental hypertension. When we examined BP effects of an isolated blockade of NO synthase by L-NAME (Protocol 2) in these three hypertensive models, we also demonstrated the relative NO deficiency in the SHRs and the DS-HS (Table 1). When we induced an isolated blockade of BKCa channels by TEA according to Protocol 3, we observed a tendency toward increased absolute BP responses and decreased relative BP responses in all three models. However, these changes did not attain statistical significance (Table 1).
Discussion
The present study aimed to evaluate how particular vasodilator systems (prostanoids, BKCa channels and NO) respond to the development of chronic BP elevation. On the basis of our long-term experience, we have chosen three reliable models of genetic, NO-deficient and salt hypertension with a similar extent of BP elevation. All three forms of experimental hypertension are characterized by an enhanced contribution of sympathetic vasoconstriction to BP maintenance. Our data revealed a compensatory activation of both prostanoids and BKCa channels in all forms of hypertension examined. The augmentation of the vasodilator activity in both systems was proportional to the degree of BP elevation because we observed an enhanced BP rise in hypertensive rats after the acute blockade of these vasodilator systems, but the relative BP rise (expressed as a percentage of the basal BP) was not significantly different from the respective normotensive controls. On the contrary, the BP response to an acute inhibition of NO formation was never enhanced in hypertensive animals compared with their controls. When the BP rise elicited by L-NAME injection was expressed in terms of basal BP, a severe relative NO deficiency was disclosed in both SHRs and DS-HS rats. Thus, NO deficiency—either relative (genetic or salt hypertension) or absolute (chronic L-NAME hypertension)—represents the principal vasodilator defect in experimental hypertension.
The activation of vascular synthesis and the release of vasodilator prostanoids (namely PGI2) were repeatedly demonstrated in rats with genetic10, 11 or salt hypertension.8, 9 It should be noted that such an activation of prostanoid formation was absent in prehypertensive SHRs11, 12 or in salt-sensitive Dahl rats fed on a low-salt diet.8, 9 This result suggests that prostanoid synthesis is enhanced as a consequence of high BP and/or underlying hypertensive mechanisms. In addition, the acute COX inhibition by indomethacin increased the vascular resistance in perfused SHRs’ hindlimbs.15, 16 Thus, our data on the enhanced indomethacin-induced BP rise in conscious hypertensive rats, as well as a highly significant positive correlation (Figure 7b) between the indomethacin-induced BP rise and the basal BP level, represent a direct confirmation of the validity of the above data under the in vivo conditions.
Interestingly, Jones et al.27 reported that norepinephrine, through its alpha1-adrenergic mechanisms, stimulated the in vitro production of PGI2, prostaglandin E2 and thromboxane A2 in the aorta of salt-loaded rats, and this prostanoid formation was effectively inhibited by indomethacin, which also shifted the contractile dose–response curve to higher norepinephrine concentrations. When we examined the relationships between sympathetic vasoconstriction and prostanoid-dependent vasodilatation in our set of experimental animals, we found a highly significant correlation between the magnitude of the pentolinium-induced BP fall and the indomethacin-induced BP rise (r=0.599, n=53, P<0.0001), which is in good agreement with the in vitro findings of Jones et al.27 There was a closer correlation between the basal BP and the pentolinium-induced BP fall than with the indomethacin-induced BP rise (Figures 7a and b), supporting the primary importance of sympathetic hyperactivity for the maintenance of high BP. Indomethacin is a non-selective COX inhibitor. Our preliminary experiments with selective COX inhibitors in SHRs indicated that combined COX1 (SC-560 or valeryl salicylate) and COX2 (celecoxib) inhibition is necessary for mimicking the observed indomethacin-induced BP changes. Nevertheless, selective COX2 inhibition had significantly greater BP effects in SHRs than in WKY rats (34.5±1.7 vs. 9.2±3.4 mm Hg). Moreover, the BP changes elicited by celecoxib corresponded to 65±2% of the indomethacin-induced BP rise, whereas it was only 36±1% in WKY rats (Behuliak, unpublished data).
PGI2, which is a dominant vasodilator prostaglandin, controls vascular tone through cAMP formation.28 Our recent studies confirmed the importance of cAMP in the control of Ca2+ entry through L-VDCCs.29, 30 It is important to note that indomethacin-induced BP changes can be effectively prevented by nifedipine pretreatment, and they can also be abolished if nifedipine is injected in animals with established indomethacin-induced BP elevation (Zicha, unpublished data). These results demonstrate the role of Ca2+ entry through L-VDCCs in the prostanoid-mediated control of vascular tone.
The activation of large conductance Ca2+-activated K+ channels (BKCa) in the vascular smooth muscle of hypertensive rats is the basis of increased K+ turnover/K+ efflux in various forms of experimental hypertension (for review see Jones;31 Furspan;32 Bohr33). BKCa channels might be activated because of the enhanced Ca2+ influx and/or the deficient activity of voltage-dependent K+ channels (for review see Cox and Rusch34). In contrast to a large number of sophisticated in vitro studies performed in isolated arteries or other tissues, there is a lack of information on their function in resistance vessels under the in vivo conditions. Using appropriate doses of TEA, which did not surpass the concentration of 0.5 mmol l−1 in the extracellular fluid, we have demonstrated major transient BP peaks, which were augmented in hypertensive rats compared with normotensive rats. This result was in a good agreement with our recent study,35 which revealed enhanced contraction of the femoral arteries isolated from SHRs in the presence of 1 mmol l−1 TEA. To verify the specificity of our BKCa channel blockade, we have performed some pilot experiments in normotensive WKY rats, in which the acute administration of a more specific BKCa channel blocker (paxilline, 5 mg kg−1 i.v.) to animals pretreated with captopril and pentolinium caused a similar BP elevation as the injection of TEA (20 mg kg−1; 20±3 vs. 24±4 mm Hg), although the paxilline-induced BP rise was less steep and more prolonged compared with that elicited by TEA.
The availability of mouse strains deficient in the function of BKCa channels (usually caused by the disruption of their β1 or α subunits) offers new tools for the analysis of the role of these channels in cardiovascular physiology. The inborn dysfunction of BKCa channels in these strains is associated with a moderate BP elevation of 5–20 mm Hg.36, 37, 38, 39 Although these mouse data suggest only a minor role of BKCa in BP regulation, all knockout models represent the animals in which the chronic deficiency of particular channels was present during the entire ontogeny and is, therefore, compensated by other available systems to a large extent. Thus, a comparison between the BPs in knockout and wild-type mice can hardly reveal the contribution of BKCa channels to the vasodilatation in normal animals.
The mechanism of BKCa channel activation might also be related to the sympathetic hyperactivity. Nelson et al.40 described the activation of L-VDCCs after norepinephrine stimulation, which results in an enhanced Ca2+ influx. Our earlier study4 demonstrated that a Ca2+ influx through L-VDCCs during the tonic phase of norepinephrine-induced vascular contraction represents a decisive part of enhanced sympathetic vasoconstriction in SHRs. Moreover, Kuneš et al.5 reported an increased nifedipine-sensitive BP component in all three forms of experimental hypertension examined in the present study. When we analyzed the relationship between sympathetic vasoconstriction and BKCa-dependent vasodilatation in our animals, we again observed a highly significant correlation between the magnitude of the TEA-induced BP rise and the magnitude of the pentolinium-induced BP fall (r=0.554, n=53, P<0.0001) or the basal BP (Figure 7c), supporting the above hypothesis on the role of the sympathetic hyperactivity and the enhanced Ca2+ influx during BKCa activation. It should also be noted that a major part of the TEA-induced BP rise can be blocked by nifedipine, suggesting that the opening of L-VDCCs resulting from the absence of the hyperpolarizing action of BKCa channels is the main mechanism of BP increase observed after acute TEA administration (Pintérová, unpublished data).
Nevertheless, NO, which seems to be the most potent among the three endogenous vasodilator systems investigated, behaves quite differently under the conditions of spontaneous or salt hypertension. In contrast to both of the above-mentioned systems (PGI2 and BKCa channels), we did not observe any signs of the activation of NO-dependent vasodilatation in these two hypertensive models, which are both characterized by enhanced sympathetic vasoconstriction. The absolute values of the BP rise elicited by acute L-NAME injection in the spontaneously or the salt hypertensive rats never surpassed the values found in the normotensive controls (Figures 6a and c). Furthermore, when L-NAME induced BP changes were expressed as a percentage of the basal BP, the relative NO deficiency was disclosed in both the forms of experimental hypertension (Figures 6d and f). The presence of relative, but not absolute, NO deficiency in these two hypertensive models was further supported by a highly significant negative correlation between the basal MAP and the relative L-NAME induced MAP changes (r=−0.747, n=47, P<0.0001), whereas absolute L-NAME induced MAP changes did not correlate with the basal MAP (r=−0.005; Figure 7d). There was no significant correlation between the pentolinium-induced BP fall and the L-NAME induced BP elevation (r=−0.012).
In conclusion, our data suggest compensatory activation of vasodilator prostanoids and Ca2+-activated K+ channels in rats with experimental hypertension, whereas NO-dependent vasodilatation was not augmented. Thus, the overall activity of the vasodilator systems failed to compensate for the augmented sympathetic vasoconstriction in hypertensive animals.
References
Zicha J, Dobešová Z, Kuneš J . Relative deficiency of nitric oxide-dependent vasodilation in salt-hypertensive Dahl rats: the possible role of superoxide anions. J Hypertens 2001; 19: 247–254.
Pecháňová O, Dobešová Z, Čejka J, Kuneš J, Zicha J . Vasoactive systems in L-NAME hypertension: the role of inducible nitric oxide synthase. J Hypertens 2004; 22: 167–173.
Kuneš J, Dobešová Z, Zicha J . Altered balance of main vasopressor and vasodepressor systems in rats with genetic hypertension and hypertriglyceridaemia. Clin Sci (Lond) 2002; 102: 269–277.
Paulis L, Líšková S, Pintérová M, Dobešová Z, Kuneš J, Zicha J . Nifedipine-sensitive noradrenergic vasoconstriction is enhanced in spontaneously hypertensive rats: the influence of chronic captopril treatment. Acta Physiol (Oxf) 2007; 191: 255–266.
Kuneš J, Hojná S, Kadlecová M, Dobešová Z, Rauchová H, Vokurková M, Loukotová J, Pecháňová O, Zicha J . Altered balance of vasoactive systems in experimental hypertension: the role of relative NO deficiency. Physiol Res 2004; 53 (Suppl 1): S23–S34.
Dobešová Z, Kuneš J, Zicha J . The altered balance between sympathetic nervous system and nitric oxide in salt hypertensive Dahl rats: ontogenetic and F2 hybrid studies. J Hypertens 2002; 20: 945–955.
Zicha J, Dobešová Z, Kuneš J . Adaptive role of nitric oxide (NO) in hypertension: relative but not absolute deficiency. Adapt Med 2009; 1: 112.
Limas C, Goldman P, Limas CJ, Iwai J . Effect of salt on prostaglandin metabolism in hypertension-prone and -resistant Dahl rats. Hypertension 1981; 3: 219–224.
Uehara Y, Tobian L, Iwai J, Ishii M, Sugimoto T . Alterations of vascular prostacyclin and thromboxane A2 in Dahl genetical strain susceptible to salt-induced hypertension. Prostaglandins 1987; 33: 727–738.
Uehara Y, Ishimitsu T, Ishii M, Sugimoto T . Prostacyclin synthase and phospholipases in the vascular wall of experimental hypertensive rats. Prostaglandins 1987; 34: 423–432.
Osanai T, Matsumura H, Kikuchi T, Minami O, Yokono Y, Akiba R, Eidou H, Konta A, Kanazawa T, Onodera K, Metoki H, Oike Y . Changes in vascular wall production of prostacyclin and thromboxane A2 in spontaneously hypertensive rats during maturation and the concomitant development of hypertension. Jpn Circ J 1990; 54: 507–514.
Ishimitsu T, Uehara Y, Ishii M, Ikeda T, Matsuoka H, Sugimoto T . Alterations of the cardiovascular and renal prostaglandins and thromboxanes system in prehypertensive spontaneously hypertensive rats. Jpn Circ J 1989; 53: 307–312.
Inglés AC, Ruiz FJ, Salom MG, Quesada T, Carbonell LF . Role of nitric oxide and prostaglandins in the regulation of blood pressure in conscious rats. Can J Physiol Pharmacol 1995; 73: 693–698.
Ruiz FJ, Inglés AC, Quesada T, Salom MG, Carbonell LF . Indomethacin does not modify the role of nitric oxide on blood pressure regulation of SHR. Gen Pharmacol 1994; 25: 103–106.
Imaizumi T, Takeshita A, Ashihara T, Nakamura M . Salt loading augments vascular responses to indomethacin in stroke-prone SHR. Am J Physiol 1982; 243: H360–H364.
Imaizumi T, Takeshita A, Ashihara T, Nakamura M . Endogenous prostaglandins do not modulate the hindquarters vascular responses to adrenergic stimulation in rats. Prostaglandins Leukot Med 1983; 10: 65–72.
Chrysant SG, Townsend SM, Morgan PR . The effects of salt and meclofenamate administration on the hypertension of spontaneously hypertensive rats. Clin Exp Hypertens 1978–1979; 1: 381–391.
Chrysant SG, Baxter PR, Almonette RL . Systemic and renal hemodynamic effects of captopril and meclofenamate in salt-treated SHR. Curr Ther Res 1983; 34: 857–868.
Nakanishi K, Chinen A, Saito Y, Hamada K, Hara N, Nagai Y . Nitric oxide buffers renal medullary vasoconstriction induced by prostaglandins synthesis blockade. Hypertens Res 2001; 24: 699–704.
Cox RH . Changes in the expression and function of arterial potassium channels during hypertension. Vasc Pharmacol 2002; 38: 13–23.
Ledoux J, Werner ME, Brayden JE, Nelson MT . Calcium-activated potassium channels and the regulation of vascular tone. Physiology 2006; 21: 69–79.
Jones AW . Altered ion transport in vascular smooth muscle from spontaneously hypertensive rats. Influences of aldosterone, norepinephrine, and angiotensin. Circ Res 1973; 33: 563–572.
Jones AW, Hart RG . Altered ion transport in aortic smooth muscle during deoxycorticosterone acetate hypertension in the rat. Circ Res 1975; 37: 333–341.
Furspan PB, Bohr DF . Lymphocyte abnormalities in three types of hypertension in the rat. Hypertension 1985; 7: 860–866.
Furspan PB, Bohr DF . Calcium sensitivity of Ca2+-activated K+ channels in spontaneously hypertensive stroke-prone rats. Hypertension 1990; 15 (Suppl I): 97–101.
Minami N, Imai Y, Hashimoto J, Abe K . Contribution of vascular nitric oxide to basal blood pressure in conscious spontaneously hypertensive rats and normotensive Wistar Kyoto rats. Clin Sci (Lond) 1995; 89: 177–182.
Jones SB, Liu Y, Jones AW . Altered aortic production of 6-keto-prostaglandin F1α from aldosterone-salt hypertensive rats. J Vasc Res 1992; 29: 256–263.
Vane JR, Botting RM . Secretory functions of the vascular endothelium. J Physiol Pharmacol 1992; 43: 195–207.
Pintérová M, Líšková S, Dobešová Z, Behuliak M, Kuneš J, Zicha J . Impaired control of L-type voltage-dependent calcium channels in experimental hypertension. Physiol Res 2009; 58 (Suppl 2): S43–S54.
Pintérová M, Karen P, Kuneš J, Zicha J . Role of nifedipine-sensitive sympathetic vasoconstriction in maintenance of high blood pressure in spontaneously hypertensive rats: effect of Gi-protein inactivation by pertussis toxin. J Hypertens 2010; 28: 969–978.
Jones AW . Ionic dysfunction and hypertension. Adv Microcirc 1982; 11: 134–159.
Furspan PB, Bohr DF . Cell membrane permeability in hypertension. Clin Physiol Biochem 1988; 6: 122–129.
Bohr DF . Cell membrane in hypertension. News Physiol Sci 1989; 4: 85–88.
Cox RH, Rusch NJ . New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation 2002; 9: 243–257.
Líšková S, Petrová M, Karen P, Kuneš J, Zicha J . Influence of calcium-dependent potassium channel blockade and nitric oxide inhibition on norepinephrine-induced contractions in two forms of genetic hypertension. J Am Soc Hypertens 2010; 4: 128–134.
Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC . Hypertension of Kcnmb1-/- is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 2009; 106: 11800–11805.
Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW . Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 2000; 407: 870–876.
Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P . Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation 2005; 112: 60–68.
Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O . Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res 2000; 87: E53–E60.
Nelson MT, Standen NB, Brayden JE, Worley JF . Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature 1988; 336: 382–385.
Acknowledgements
The valuable comment of Ivana Vaněčková is highly appreciated. The experimental work was partially supported by AV0Z 50110509 and research Grants GA CR 305/09/0336, IGA AV CR IAA500110902 and 1M0510 (Ministry of Education of the Czech Republic).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Behuliak, M., Pintérová, M., Kuneš, J. et al. Vasodilator efficiency of endogenous prostanoids, Ca2+-activated K+ channels and nitric oxide in rats with spontaneous, salt-dependent or NO-deficient hypertension. Hypertens Res 34, 968–975 (2011). https://doi.org/10.1038/hr.2011.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.82
Keywords
This article is cited by
-
Experimental preeclampsia in rats affects vascular gene expression patterns
Scientific Reports (2017)