Abstract
Statins exert pleiotropic effects, including antioxidative and cellular protective effects. Endogenous adrenomedullin (AM) induces anti-inflammatory, anti-fibrotic and proangiogenic effects. We examined the effects of simvastatin on cardiac fibrosis and apoptosis in AM heterozygous knockout (AM+/−) mice treated with angiotensin (Ang) II and high salt loading. Seven-week-old AM+/− mice were infused with Ang II while on a high-salt diet with or without simvastatin for 2 weeks. Hearts were stained by hematoxylin-eosin or Masson's trichrome, and were immunostained with isolectin B4 and α-smooth muscle actin antibodies. Expression of c-Kit and Sca-1 messenger RNA (mRNA) was evaluated by real-time PCR analysis. Apoptotic cells in hearts were identified by terminal deoxynucleotidyl transferase-mediated UTP end labeling (TUNEL) staining. Hearts from Ang II/salt loading AM+/− mice showed marked perivascular fibrosis around coronary arteries. Treatment with simvastatin significantly inhibited the fibrosis around coronary arteries in Ang II/salt-loading AM+/− mice. Expression of c-Kit and Sca-1 mRNAs in hearts from Ang II/salt-loading AM+/− mice was significantly lower than in hearts from wild-type mice. Treatment with simvastatin significantly increased the suppressed expression of c-Kit and Sca-1 mRNAs. In addition, treatment with simvastatin significantly increased the number of isolectin B4-positive capillary arteries in hearts from Ang II/salt-loading AM+/− mice. Ang II/high salt significantly increased apoptotic cells in hearts from AM+/− mice; this trend was reversed by treatment with simvastatin. Thus, statins have potent cardioprotective effects that may be associated with anti-fibrotic, proangiogenic and anti-apoptotic effects in Ang II/salt-loading AM+/− mice.
Similar content being viewed by others
Introduction
Adrenomedullin (AM) is a ubiquitously expressed multifunctional peptide that exerts vasodilatory, hypotensive1 and neuromodulatory effects;2 positive inotropic effects on cardiomyocytes;3 natriuretic,4 anti-apoptotic5 and antioxidative stress effects;6 and proangiogenic effects.7 As AM is a pivotal intrinsic antioxidative substrate in tissues, AM insufficiency is associated with the pathogenesis of cardiovascular disease, hypertension, diabetes and renal disease. It has been reported that angiotensin II (Ang II) plus high salt (Ang II/salt) loading induce cardiac fibrosis in AM heterozygous knockout (AM+/−) mice and that AM inhibits cardiac fibrosis in Ang II-induced hypertensive rats.8, 9 Thus, Ang II/salt loading in the AM+/− mouse is a useful model for cardiovascular damage, including cardiac fibrosis.
Statins have pleiotropic effects independent of their cholesterol-lowering effects.10, 11 Statins induce potent anti-oxidative effects, such as increases in the antioxidative molecule, hemoxidase-1 (HO-1).12 Statins have also been reported to exert potent cardioprotective effects independent of their cholesterol-lowering effects.10 Statins effectively improve cardiac function and increase the survival rate in patients with severe cardiac failure due to dilated cardiomyopathy without coronary arterial insufficiency.13, 14 In addition, statins upregulate and activate endothelial nitric oxide synthase (eNOS) and increase NO production through the PI3K-Akt-eNOS pathway.15, 16 Statins inhibit the formation of mevalonate and exhibit powerful anti-inflammatory effects, mediated by the inhibition of critical proteins such as Ras, Rho and NF-κB.17 Thus, the anti-inflammatory effects of statins induce potent cardioprotective effects through the inhibition of Rho kinase.18
To further investigate the mechanisms underlying the cardioprotective effects of statin, we examined the effects of simvastatin on cardiac fibrosis and apoptosis in AM+/− mice treated with Ang II and high-salt diet.
Methods
Our investigation conformed to the standards of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The ethics committee of Nihon University School of Medicine examined every research protocol involving the use of living animals.
Animals
Adrenomedullin heterozygous knockout C57BL/6 male (AM+/−) mice and C57BL/6 male (wild-type) mice were purchased from a laboratory at Tokyo University and used in this study.
Treatments for AM+/− mice
Seven-week-old AM+/− mice were infused with Ang II (640 ng kg−1 min−1, Peptide Institute, Osaka, Japan) using osmotic mini-pumps (Alza, Cupertino, CA USA) while on a high-salt diet (8% NaCl) with or without oral simvastatin treatment (Banyu Pharmaceuticals, Tokyo, Japan) (40 mg kg−1 per day) for 2 weeks.
Histological examination
At 2 weeks after the start of simvastatin treatment, hearts were removed from mice and embedded in paraffin. Paraffin-embedded sections obtained from each segment were stained with hematoxylin-eosin stain and Masson's trichrome. The fibrous area was determined on the basis of the representative Masson's trichrome-stained area using Photoshop CS3 extended (Adobe, Tokyo, Japan). Vessel formation was immunostained with α-smooth muscle actin (α-SMA) (DAKO, Tokyo, Japan) and isolectin B4 antibodies. Biotinylated isolectin B4 (Vector Laboratories, Burlingame, CA, USA) and streptavidin-FITC (Vector Laboratories) were used for microvessel immunostaining. Nuclei were stained with Hoechst 33342 (Sigma-Aldrich, St Louis, MO, USA). Myocyte number was determined counting the nuclei of isolectin B4- and α-SMA-negative cells. Apoptotic cells were determined by terminal deoxynucleotidyl transferase-mediated UTP end labeling (TUNEL) staining using an apoptosis detection kit (Takara, Shiga, Japan).
RNA extraction and PCR
Total RNA was extracted from mouse hearts using a Trizol reagent (Invitrogen, CA, USA). Total RNA (1 μg) was reverse transcribed into complementary DNA with random 9-mers using a Takara RNA PCR Kit (AMV) Ver. 3.0 (Takara Bio, Ohtsu, Japan). Assay-on-demand primers and probes (c-Kit: Mm00445212_m1, Sca-1: Mm00726565_s1 and TaqMan Rodent GAPDH control reagents) were purchased from Applied Biosystems. Messenger RNA (mRNA) was quantified with an ABI Prism 7300 (Applied Biosystems, Carlsbad, CA, USA). Each sample (each reaction, 5 μl complementary DNA; total volume, 25 μl) was run in triplicate. Cycling parameters were 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. After confirming that the efficiencies of the target and the endogenous control amplifications were approximately equal, relative gene expression was analyzed using the comparative ΔΔCt method with GAPDH as the endogenous control.
Measurement of 8-iso-prostaglandin F2α (8-iso-PGF2α)
Mice were placed in metabolic cages to collect urine for 24 h after the infusion of Ang II with high-salt diet and oral treatment of simvastatin for 2 weeks. Urinary 8-iso-PGF2α was measured by enzyme-linked immunoassay (Oxford Biomedical Research, Oxford, UK).
Statistical analysis
Statistical analysis was performed using the unpaired Student's t-test and Welch's t-test. Data were expressed as the mean±standard error of the mean (s.e.m.). Differences were considered significant at the level of P<0.05.
Results
Effects of simvastatin on cardiac fibrosis
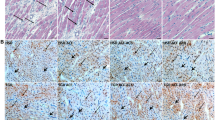
Ang II/high salt loading induced marked cardiac fibrosis in the left ventricle (Figure 1) and around coronary arteries (Figure 2a) in AM+/− mice but not in wild-type mice. Cardiac fibrosis was quantitatively analyzed by measuring the extent of perivascular fibrosis around coronary arteries. The area of perivascular fibrosis was significantly higher (P<0.05) in hearts from AM+/− mice. This effect was significantly (P<0.05) inhibited by simvastatin treatment (Figure 2b).
Cardiac fibrosis in hearts from adrenomedullin heterozygous knockout (AM+/−) mice infused with angiotensin II (Ang II) and submitted to salt loading. Seven-week-old wild-type or AM+/− mice were infused with Ang II (640 ng kg−1 min−1) on an 8% high-salt diet. Hearts were removed from mice and embedded in paraffin and were stained with hematoxylin-eosin and Mason's trichrome stains.
(a) Cardiac fibrosis around coronary arteries in left ventricles from adrenomedullin heterozygous knockout (AM+/−) mice treated with angiotensin II (Ang II) and salt loading. Seven-week-old wild-type or AM+/− mice were infused with Ang II (640 ng kg−1 min−1) on an 8% high-salt diet with oral simvastatin treatment (40 mg kg−1 per day). (b) The fibrous area was identified as the Masson's trichrome-stained area on each NIH image. Data are the mean±s.e.m. (n=4). *P<0.05 between indicated columns.
Effects of simvastatin on expression of c-Kit and Sca-1 mRNA
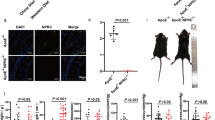
Figures 3a and b show effects of simvastatin on mRNA expression of c-Kit and Sca-1 in hearts from wild or AM+/− mice treated with or without Ang II/high salt. The abundance of c-Kit and Sca-1 mRNAs in hearts from Ang II/salt-loading AM+/− mice was significantly (P<0.05) lower than in hearts from wild-type mice. Treatment with simvastatin significantly (P<0.05) increased the abundance of c-Kit as well as Sca-1 mRNA in hearts from Ang II/salt-loading AM+/− mice.
Effects of simvastatin on mRNA expression of the oxidative stress marker 8-iso-PGF2α, and CSC markers c-Kit and Sca-1 in the hearts of wild or adrenomedullin heterozygous knockout (AM+/−) mice treated with angiotensin (Ang) II and high salt loading. Seven-week-old wild-type or AM+/− mice were infused with Ang II (640 ng kg−1 min−1) on an 8% high-salt diet and oral treatment with simvastatin (40 mg kg−1 per day). Total RNA was extracted from mouse hearts. Expression of c-Kit (a) and Sca-1 (b) mRNA was evaluated by real-time PCR analysis. Relative gene expression was analyzed by the comparative ΔΔCt method with GAPDH. (c) Levels of the oxidative stress marker, 8-iso-PGF2α, in mouse urine were measured by ELISA. Data are the mean±s.e.m. (n=4). *P<0.05 between indicated columns.
Effects of simvastatin on oxidative stress in AM+/− mice
Figure 3c shows the effects of simvastatin on expression of the oxidative stress marker 8-iso-PGF2α in urine from wild or AM+/− mice treated with or without Ang II/high salt. The excretion of 8-iso-PGF2α in urine from Ang II/salt-loading AM+/− mice was higher (but not significantly) than in urine from Ang II/salt-loading wild-type mice. Treatment with simvastatin decreased urinary excretion of 8-iso-PGF2α in Ang II/salt-loading AM+/− mice.
Angiogenic effects of simvastatin
Figure 4 shows immunohistochemical staining for isolectin B4 and α-SMA in the left ventricles of hearts from wild-type and AM+/− mice treated with Ang II/high salt and simvastatin. The capillary density of the ventricle was significantly (P<0.05) lower in hearts from Ang II/salt-loading AM+/− mice than in Ang II/salt-loading wild-type mice. Treatment with simvastatin significantly increased the number of isolectin B4-positive (P<0.05) and α-SMA-positive (P<0.01) capillaries in the ventricles of hearts from Ang II/salt-loading AM+/− mice.
Angiogenic effects of simvastatin on hearts from wild or adrenomedullin heterozygous knockout (AM+/−) mice treated with or without angiotensin (Ang) II and high salt loading. Seven-week-old wild-type or AM+/− mice were infused with or without Ang II (640 ng kg−1 min−1) on an 8% high-salt diet with or without oral treatment with simvastatin (40 mg kg−1 per day). After 2 weeks, hearts were removed from mice and embedded in paraffin. (a) Vessels were immunostained with α-smooth muscle actin (α-SMA) and isolectin B4 antibodies. High magnification insets in each image. (b) Number of capillaries in each field ( × 200). Scale bar indicates 100 μm. Data are expressed as mean±s.e.m. (n=4). Data are the mean±s.e.m. (n=4). *P<0.05, **P<0.01 between indicated columns.
Anti-apoptotic effects of simvastatin
Figure 5 shows TUNEL staining in the left ventricles of hearts from wild-type and AM+/− mice treated with Ang II/high salt and simvastatin. The percentage of apoptotic cells in the ventricle was significantly (P<0.05) higher in hearts from AM+/− mice as compared with wild-type mice. Treatment with simvastatin significantly (P<0.05) decreased the percentage of apoptotic cells in the ventricles of hearts from Ang II/salt-loading AM+/− mice. Treatment with simvastatin did not appreciably affect the number of myocytes in the ventricles of hearts from AM+/− mice treated with Ang II/high salt.
Anti-apoptotic effects of simvastatin on hearts from wild or adrenomedullin heterozygous knockout (AM+/−) mice treated with or without angiotensin (Ang) II and high salt loading. Seven-week-old wild type or AM+/− mice were infused with or without Ang II (640 ng kg−1 min−1) on an 8% high-salt diet with or without oral treatment with simvastatin (40 mg kg−1 per day). After 2 weeks, hearts were removed from mice and embedded in paraffin. (a) Apoptotic cells were identified by terminal deoxynucleotidyl transferase-mediated UTP end labeling (TUNEL) staining (brown). (b) Apoptotic cell percentage was calculated as the number of TUNEL-positive cells divided by the total number of nuclei ( × 200). Data are the mean±s.e.m. (n=8). *P<0.05 between indicated columns.
Discussion
To investigate mechanisms of the cardioprotective effects of statin, we examined effects of simvastatin on fibrosis, apoptosis and angiogenesis in hearts from AM+/− mice treated with Ang II and high salt. AM+/− mice showed marked cardiac fibrosis in heart muscle with Ang II/salt-induced oxidative stress. Treatment with simvastatin significantly repaired the cardiac fibrosis and decreased levels of 8-iso-PGF2α in hearts from Ang II/salt-loading AM+/− mice, suggesting that simvastatin inhibited cardiac fibrosis through its antioxidative actions.
Moreover, treatment with simvastatin significantly increased capillary density, as determined by immunostaining with isolectin B4 and α-SMA in hearts from Ang II/salt-loading AM+/− mice, suggesting that simvastatin strongly increased mature capillary arteries comprising endothelium and vascular smooth muscle. We recently demonstrated that atorvastatin induces proangiogenic effects, with increase in the expression of angiogenic factors, including vascular endothelial growth factor, interluekin-8, angiopoietins and eNOS, in the ischemic hindlimbs of rats.19 Statins have been reported to increase HO-1 production and, thus, inhibit oxidation in vivo.12 Hsu et al.20 demonstrated that oral administration of statins increases HO-1 production in the mouse liver, lung, brain and heart, and suggested that the antioxidative effects of statins in cardiovascular tissues are strongly induced by HO-1. Moreover, we demonstrated that a statin strongly potentiates colony formation among endothelial progenitor cells during angiogenesis and repairs the endothelium in oxidative hypertensive rats with increases in HO-1 in vivo.19 It is possible that the proangiogenic effects of simvastatin also protected against cardiac fibrosis. Suzuki et al.21 recently demonstrated that pravastatin mobilizes bone marrow progenitor cells in hibernating hearts and increases the number of myocytes that reenter the growth and mitotic phases of the cell cycle. In this experiments, simvastatin did not increase the number of myocytes; however, simvastatin decreased the number of apoptotic cells in hearts from Ang II/salt-loading AM+/− mice, indicating that the cardioprotective effects of simvastatin are associated with anti-apoptotic effects.
The presence of cardiac stem cells in the heart has recently been reported. Beltrami et al.22 first reported the discovery of a distinct resident population of cardiac stem cells that express c-Kit, the receptor for stem cell factor. C-Kit, the transmembrane tyrosine kinase receptor for stem cell factor, is required for melanocyte and mast cell development, hematopoiesis and the differentiation of spermatogonial stem cells.23 c-Kit is also transiently expressed in cardiomyocyte precursors during development and in a rare cell population in the normal adult heart. Li et al.24 demonstrated that in the heart, c-Kit is expressed not only by cardiac stem cells but also by cardiomyocytes. Expression is observed immediately after birth and terminates a few days later and, thus, coincides with the onset of cardiomyocyte terminal differentiation. c-Kit expression in the heart has also been implicated in mediating repair and remodeling after myocardial infarction as well as in the maintenance of cardiac function.25 Thus, c-Kit is a marker of stem and progenitor cells as well as the regeneration of damaged heart tissue. We previously examined the effects of an Ang II-receptor blocker on the expression of c-Kit in hearts from hypertensive rats. Expression of c-Kit mRNA was significantly lower than in normotensive rats. The Ang II-receptor blocker significantly increased expression of c-Kit through antioxidative mechanisms.26 In this experiments, the abundance of c-Kit and Sca-1 mRNA in hearts from AM+/− mice treated with Ang II/high salt was significantly lower than in heart tissue from wild-type mice. Treatment with simvastatin significantly increased the abundance of c-Kit and Sca-1 mRNA in hearts from AM+/− mice treated with Ang II/high salt. These findings suggest that simvastatin inhibited cardiac fibrosis through the regeneration of damaged heart and/or activation of cardiac stem cells through antioxidative actions in Ang II/salt-loading AM+/− mice.
In conclusion, statins have potent cardioprotective effects that may be associated with anti-fibrotic, proangiogenic and anti-apoptotic effects in Ang II/salt-loading AM+/− mice.
References
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T . Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993; 192: 553–560.
Katoh F, Niina H, Kitamura K, Ichiki Y, Yamamoto Y, Kangawa K, Eto T, Wada A . Ca(2+)-dependent cosecretion of adrenomedullin and catecholamines mediated by nicotinic receptors in bovine cultured adrenal medullary cells. FEBS Lett 1994; 348: 61–64.
Sato A, Canny BJ, Autelitano DJ . Adrenomedullin stimulates cAMP accumulation and inhibits atrial natriuretic peptide gene expression in cardiomyocytes. Biochem Biophys Res Commun 1997; 230: 311–314.
Israel A, Diaz E . Diuretic and natriuretic action of adrenomedullin administered intracerebroventricularly in conscious rats. Regul Pept 2000; 89: 13–18.
Kato H, Shichiri M, Marumo F, Hirata Y . Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology 1997; 138: 2615–2620.
Yoshimoto T, Fukai N, Sato R, Sugiyama T, Ozawa N, Shichiri M, Hirata Y . Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology 2004; 145: 3331–3337.
Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, Yanagi K, Hiraoka-Kanie M, Inoue E, Ara T, Nagasawa T, Just U, Nakao K, Nishikawa S, Yamashita JK . Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol 2006; 26: 1977–1984.
Shimosawa T, Shibagaki Y, Ishibashi K, Kitamura K, Kangawa K, Kato S, Ando K, Fujita T . Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation 2002; 105: 106–111.
Tsuruda T, Kato J, Hatakeyama K, Masuyama H, Cao YN, Imamura T, Kitamura K, Asada Y, Eto T . Antifibrotic effect of adrenomedullin on coronary adventitia in angiotensin II-induced hypertensive rats. Cardiovasc Res 2005; 65: 921–929.
Stancu C, Sima A . Statins: mechanism of action and effects. J Cell Mol Med 2001; 5: 378–387.
Morikawa S, Takabe W, Mataki C, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T . Global analysis of RNA expression profile in human vascular cells treated with statins. J Atheroscler Thromb 2004; 11: 62–67.
Lee TS, Chang CC, Zhu Y, Shyy JY . Simvastatin induces hemo oxygenase-1: a novel mechanism of vessel protection. Circulation 2004; 110: 1296–1302.
Node K, Fujita M, Kitakaze M, Hori M, Liao JK . Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation 2003; 108: 839–843.
Mozaffarian D, Nye R, Levy WC . Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol 2004; 93: 1124–1129.
Laufs U, La Fata V, Plutzky J, Liao JK . Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998; 97: 1129–1135.
Bell RM, Yellon DM . Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol 2003; 41: 508–515.
Hernández-Presa MA, Ortego M, Tuñón J, Martín-Ventura JL, Mas S, Blanco-Colio LM, Aparicio C, Ortega L, Gómez-Gerique J, Vivanco F, Egido J . Simvastatin reduces NF-kappa B activity in peripheral mononuclear and in plaque cells of rabbit atheroma more markedly than lipid lowering diet. Cardiovasc Res 2003; 57: 168–177.
Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK . Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 2004; 24: 1842–1847.
Matsumura M, Fukuda N, Kobayashi N, Umezawa H, Takasaka A, Matsumoto T, Yao E-H, Ueno U, Negishi N . Effects of atorvastatin on angiogenesis in hindlimb ischemia and endothelial progenitor cell formation in rats. J Atheroscler Thromb 2009; 16: 319–326.
Hsu M, Muchova L, Morioka I, Wong RJ, Schröder H, Stevenson DK . Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun 2006; 343: 738–744.
Suzuki G, Iyer V, Cimato T, Canty Jr JM . Pravastatin improves function in mibernating myocardium by mobilizing CD133+ and cKit+ bone marrow progenitor cells and promoting myocytes to reenter the growth phase of the cardiac cell cycle. Circ Res 2009; 104: 255–264.
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P . Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003; 114: 763–776.
Pallante BA, Duignan I, Okin D, Chin A, Bressan MC, Mikawa T, Edelberg JM . Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age-dependent paracrine mechanisms. Circ Res 2007; 100: e1–e11.
Li M, Naqvi N, Yahiro E, Liu K, Powell PC, Bradley WE, Martin DIK, Graham RM, Dell'Italia LJ, Husain A . c-Kit is required for cardiomyocyte terminal differentiation. Circ Res 2008; 102: 677–685.
Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A . Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 2004; 94: 514–524.
Yu Y, Fukuda N, Yao E-H, Matsumoto T, Kobayashi N, Suzuki R, Tahaira Y, Ueno T, Matsumoto K . Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension. Am J Hypertens 2008; 21: 72–77.
Acknowledgements
This work was supported in part by a Grant-in Aid to the High-Tech Research Center from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Yamamoto, C., Fukuda, N., Jumabay, M. et al. Protective effects of statin on cardiac fibrosis and apoptosis in adrenomedullin-knockout mice treated with angiotensin II and high salt loading. Hypertens Res 34, 348–353 (2011). https://doi.org/10.1038/hr.2010.243
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.243
Keywords
This article is cited by
-
Simvastatin Significantly Reduced Alcohol-Induced Cardiac Damage in Adolescent Mice
Cardiovascular Toxicology (2024)
-
Inhibition of Angiotensin II‐Induced Cardiac Fibrosis by Atorvastatin in Adiponectin Knockout Mice
Lipids (2017)
-
Aldosterone Does Not Contribute to Renal p21 Expression During the Development of Angiotensin II-Induced Hypertension in Mice
American Journal of Hypertension (2012)