Abstract
The efficacy of antihypertensive treatment was investigated in pregnant women with chronic hypertension (CH) or gestational hypertension (GH) on the basis of the occurrence of pregnancy complications and adverse birth outcomes. Medically recorded pregnancy complications and birth outcomes of 1579 pregnant women with CH and 1098 pregnant women with GH were compared to 34 633 pregnant women without CH, GH, preeclampsia–eclampsia or any secondary hypertension who delivered newborn infants without defects in the population-based data set of the Hungarian Case–Control Surveillance System of Congenital Abnormalities, 1980–1996. Of 1579 (4.1%) pregnant women with CH, 1522 (96.4%) were treated with antihypertensive drugs. Of 1098 (2.9%) pregnant women with GH, 657 (59.8%) were treated. Pregnant women with treated CH had a higher risk of threatened abortion, preterm delivery, and placental disorders, in addition to low-birthweight newborns. However, pregnant women with untreated CH and GH had no higher risk of pregnancy complications or adverse pregnancy outcomes. Antihypertensive treatments were not able to neutralize the harm of severe CH in pregnant women, the antihypertensive treatments were not appropriate and/or effective, or related drug treatments may contribute to these adverse effects.
Similar content being viewed by others
Introduction
Hypertension is one of the most common chronic medical conditions and is harmful to the structure and function of the heart and arteries. In addition, hypertension can accelerate the development of arteriosclerosis. The commonly accepted definition of hypertension is blood pressure exceeding 140/90 mm Hg for adults aged 18 years or older.1
Hypertension is present before and during pregnancy in 1–5% of women.2, 3 The classification of hypertension based on its origin differentiates primary, or essential, hypertension and secondary hypertension. About 95% of patients with essential hypertension are caused by the interaction of polygenic liability and hazardous environmental factors, whereas secondary hypertension is caused by definable causes such as chronic kidney disease, renal artery stenosis, coartation of the aorta, Cushing's disease, and so on. A classification system of hypertension in pregnant women distinguishes four categories: chronic hypertension (CH), preeclampsia–eclampsia, preeclampsia superimposed on CH and gestational hypertension (GH).4 There are well-known guidelines for the treatment of hypertensive patients;5 however, the care of pregnant women with hypertension shows some differences from the standard care because of the possible teratogenic and/or fetotoxic effects of some drugs.3
The efficacy of drug treatments in hypertensive pregnant women has been rarely studied. There is incomplete evidence that the benefits of specific classes of antihypertensive drugs extend beyond lowering blood pressure. Therefore, the aims of our study were to evaluate adverse pregnancy/birth outcomes, that is, preterm births, low-birthweight newborns and pregnancy complications of women with CH and GH, as indicators of the efficacy of their related drug treatments in the population-based data set of newborn infants without birth defects in the Hungarian Case–Control Surveillance of Congenital Abnormalities (HCCSCA).6 Structural birth defects such as congenital abnormalities will be evaluated in a separate case–control study.
Methods
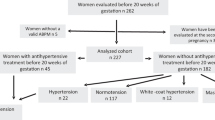
Newborn infants without congenital abnormalities were selected from the National Birth Registry of the Central Statistical Office for the HCCSCA, 1980–1996. These newborns were controls of cases with congenital abnormalities, which were selected from the Hungarian Congenital Abnormality Registry7 for the HCCSCA. In general, two newborn controls were matched individually to each case according to sex, week of birth and district of parents’ residence. The data set of the HCCSCA, 1980–1996, contains 22 843 cases with congenital abnormalities; however, many cases were excluded from this analysis because congenital abnormalities may have a more drastic effect on pregnancy complications and birth outcomes than hypertension.
Immediately after the selection of newborns, an explanatory letter was sent to the mothers explaining the purpose of the HCCSCA, and they were asked to send us the prenatal maternity logbook and every other medical record regarding their pregnancy complications and diseases during the study pregnancy for 3 weeks. Prenatal care was mandatory for pregnant women in Hungary (if somebody did not visit prenatal care clinic, she did not receive a maternity grant and leave), so nearly 100% of pregnant women visited prenatal care clinics, an average seven times during their pregnancies. The task of obstetricians was to record all pregnancy complications, other maternal diseases and related drug prescriptions in the prenatal maternity logbook during the study period.
A structured questionnaire with a list of medicines (drugs and pregnancy supplements), maternal diseases (including hypertension) and pregnancy complications, in addition to an informed consent sheet, was also mailed to the mothers. The questionnaire requested information on maternal personal (for example, employment status) and medical data, including pregnancy complications, maternal diseases and medicine intake during the study pregnancy by gestational month. To standardize the answers, mothers were asked to read the enclosed lists as a memory aid before they replied, and to send back the filled-in questionnaire and informed consent with their signature in our prepaid envelope.
The interval between the end of pregnancy and return of the information package, including prenatal maternity logbook, questionnaire, and so on was 5.2±2.9 months. In addition, 200 non-respondent and 600 respondent mothers were visited at home by regional nurses as part of two validation studies.8, 9 Regional nurses helped mothers to fill in the questionnaire used in the HCCSCA, evaluated the available medical documents (prenatal maternity logbook, discharge summary, and so on) and obtained data regarding the lifestyle (the number of daily cigarettes and drinking habit) of mothers during the study pregnancy through a cross-interview of mothers and fathers.
Overall, the necessary information was available on 83.0% of pregnant women (80.9% from reply and 2.1% from home visit). Here, the 17 years of HCCSCA data between 1980 and 1996 were evaluated including 38 151 controls because the method of data collection changed in 1997 (all mothers are visited by regional nurses), and this part of the data set has not been validated.
The evaluation of pregnant women with hypertension had four steps. First, pregnant women with secondary hypertension were excluded from the study. Second, pregnant women with preeclampsia–eclampsia (both superimposed and new onset) were also excluded. This left pregnant women with CH and new-onset GH to be included in the study. CH in pregnant women is defined as hypertension that is present and observable before pregnancy or that is diagnosed before the 20th week of gestation. GH is detected for the first time during the pregnancy in question, without proteinuria until the end of the pregnancy. In general, GH is diagnosed after the 20th gestational week10, 11 because there is a marked early decrease in diastolic and systolic blood pressures (average 7 mm Hg) because of systematic vascular resistance during pregnancy.1
Third, the preliminary analysis of our pregnant women showed that two groups of CH and GH could be differentiated according to the source of information: (i) prospectively and medically recorded CH or GH in the prenatal maternity logbook and (ii) CH or GH based on only retrospective maternal information. The medically recorded diagnosis of CH or GH was based on the available medical documents and blood pressure measured in prenatal clinic of pregnant women. In general, the concrete systolic/diastolic blood pressure values were recorded in the prenatal maternity logbook, but unfortunately we did not copy the all of the changing values; rather, we recorded only the diagnosis of CH or GH. The validity of the CH and GH diagnoses based on maternal information could not be verified; in addition, sometimes it was not possible to differentiate the different categories of hypertension, so we decided to evaluate only prospectively recorded CH and GH by obstetricians in the prenatal maternity logbook.
Fourth, the severity of CH was considered on the basis of related drug treatments. According to the classification of hypertension by severity, Stage 1 (140–159/90–99 mm Hg) and Stage 2 (160/100 mm Hg or higher) have been differentiated.4 There are several guidelines for the level of blood pressure control during pregnancy.2, 3, 5, 12, 13 Pregnant women with Stage 1 hypertension are not recommended to receive drug treatment, dietary modification (particularly sodium reduction1) or more intensive physical activity. In addition, maternal weight gain control is thought to suffice by experts on the topic.3 The initiation of antihypertensive drug therapy is usually considered in pregnant women with Stage 2 hypertension, that is, when systolic blood pressure exceeds 160 mm Hg or diastolic pressure exceeds 100 mm Hg.
Related drug treatments were evaluated based on both medically recorded data and maternal information in the questionnaire. Other potential confounding factors included maternal age, birth order, marital and employment status as indicators of socio-economic status,14 other maternal diseases, and pregnancy supplements, particularly folic acid and multivitamins, as indicators of the standard of preconception and prenatal care.
Pregnancy complications were evaluated on the basis of medical records in the prenatal maternity logbook. Both birth weight and gestational age at delivery were medically documented in the discharge summary of mothers after delivery because all deliveries took place in inpatient obstetric clinics in Hungary and the birth attendants were obstetricians. Gestational age was calculated from the first day of the last menstrual period. The definition of preterm birth was less than 37 completed weeks (less than 259 days), whereas the definition of low-birthweight newborns was less than 2500 g.
Statistical methods
We used SAS version 8.02 (SAS Institute, Cary, NC, USA) for statistical analyses. The occurrence of CH and GH separately was compared with the reference group of all pregnant women without hypertension, meaning pregnant women with CH or GH in addition to secondary hypertension or preeclampsia–eclampsia were excluded from the reference group. Contingency tables were prepared for the main study variables. First, the characteristics of pregnant women with CH or GH were compared with the reference group using the χ2-test for categorical variables or Student's t-test for quantitative variables. Second, other maternal diseases and hypertension-related drug treatments were evaluated. Third, frequency of pregnancy complications was compared between mothers with CH or GH and the reference group by ordinary logistic regression models and odds ratios with 95% confidence intervals. Finally, the birth outcomes of mean gestational age at delivery, birth weight, preterm births and low-birthweight newborns were compared in mothers with CH or GH with the reference group using the adjusted Student's t-test and odds ratios with 95% confidence intervals.
Results
The total number of births in Hungary was 2 146 574 during the study period of 1980–1996. Therefore, the 38 151 newborn infants represented 1.8% of all Hungarian births, and 1579 (4.1%) pregnant women were diagnosed with CH, whereas 1098 (2.9%) had a medically recorded diagnosis of GH in the prenatal maternity logbook. GH was diagnosed after the 20th gestational week in nearly all pregnant women.
Of 1579 pregnant women with CH, 1522 (96.4%) were treated with antihypertensive drugs and were thus classified as Stage 2. Of 1098 mothers with GH, 657 (59.8%) were treated with antihypertensive drugs; therefore, ∼40% of pregnant women with GH had Stage 1 hypertension.
The main variables of mothers with CH or GH with or without related drug treatments were compared with the reference group of 34 633 pregnant women (Table 1). Pregnant women with treated CH and GH had a higher average age than the reference group. However, the mean maternal age was somewhat lower in pregnant women with GH and particularly with CH without antihypertension treatment compared with the mean age of the reference group. The mean birth order was lower in pregnant women with treated CH and GH and even lower in pregnant women with untreated CH and GH compared with the reference group. The distribution of employment status was different in the group of pregnant women with treated CH and GH compared with the reference group. The proportion of folic acid supplementation during pregnancy was greater in pregnant women with CH and smaller in pregnant women with GH compared with the reference group. Of 800 mothers visited at home, 36 had CH, and among them 8 (22.2%) were smokers, whereas of the 764 mothers without CH, 144 (18.8%) smoked during the study pregnancy.
Obvious differences were seen in the frequency of drugs used for the treatment of CH and GH in pregnant women and in the reference group (Table 2). However, the distribution of these drugs was different in the group of CH and GH mothers. The most common drug used was terbutaline, followed by verapamil, metoprolol, nifedipin, methyldopa, fenoterol and Panangin in the CH group, whereas this order was methyldopa, clopamide, dihydralazine, nifedipin and metoprolol for the GH group. The drugs shown in Table 2 were taken by some reference pregnant women as well, but in each case the indication was different.
The secondary aim of the study was the evaluation of pregnancy complications (except preeclampsia–eclampsia, which was excluded from this analysis) in women with CH or GH (Table 3). There are obvious differences in the occurrences of some pregnancy complications between pregnant women with CH and those with GH. The rates of threatened abortion, placental disorders (mainly premature separation of placenta, that is, placental abruption) and, particularly, threatened preterm delivery were higher in pregnant women with treated CH compared to the other groups, whereas these variables among pregnant women with untreated CH or GH did not differ from the reference values. However, gestational diabetes was higher in both treated CH and GH groups.
The primary aim of the study was the evaluation of birth outcomes (Table 4). There was no difference in the sex ratio among the study groups. The mean gestational age at delivery was shorter by 0.5 week, and the mean birth weight was lower by 136 g in the treated CH subgroup than in the reference group. On the other hand, the limited number of newborns of untreated pregnant women with CH had longer mean gestational ages and larger mean birth weights. In agreement with these quantitative variables, the rates of preterm births and low-birthweight newborns of pregnant women with treated CH were much higher than in untreated women with CH. The mean gestational age at delivery was similar in the groups of pregnant women with treated and untreated GH and was 0.2 week shorter than in the reference group. The mean birth weight was similar from mothers with GH (either treated or not treated) and the reference babies. In addition, the rate of preterm births by the GH mothers (treated or untreated) did not differ significantly from the values of the reference group, but the rate of low-birthweight newborns was marginally higher in the subgroup of pregnant women with treated GH.
Finally, there was a higher rate of twins in the subgroups of pregnant women with treated GH (2.4%) and with treated CH (3.1%) than in the reference group (1.0%). However, the aforementioned higher rate of preterm births and low-birthweight newborns was found after the exclusion of twins but to a lesser degree.
Discussion
The aim of the study was to evaluate the efficacy of antihypertensive treatment of pregnant women with CH and GH on the basis of the incidence of pregnancy complications and adverse birth outcomes. The severe CH that required antihypertensive treatment was associated with a higher risk of threatened abortion, preterm delivery, placental disorders and gestational diabetes in pregnant women; in addition, they had a higher rate of preterm births and low-birthweight newborns. However, GH with or without treatment did not show a higher risk for preterm births, though the rate of low-birthweight newborns was somewhat higher in the subgroup of pregnant women with treated GH. Among pregnancy complications, only gestational diabetes occurred more frequently in pregnant women with treated GH.
The prevalence of CH and GH was 4.1 and 2.9% in Hungarian pregnant women, respectively. Our data also show that CH and GH occur more frequently in mothers of advanced age. Pregnant women with GH had a significantly lower rate of folic acid and multivitamin supplement use, it would be necessary to study whether these factors contribute to the origin of GH or not.15 The incidence of gestational diabetes was higher in pregnant women with either treated CH or treated GH.
The major benefit of antihypertensive therapy is the expected reduction pregnancy complications, particularly preeclampsia–eclampsia and adverse birth outcomes.16 (We did not find an effective preeclampsia–eclampsia preventive effect of antihypertensive treatments in another study.) The main result of our study is that antihypertensive treatments were not able to prevent the higher risk of pregnancy complications and adverse birth outcomes in pregnant women with Stage 2 CH.
For antihypertensive therapy in pregnant women, in general methyldopa is the first choice. Its safety has been shown in several reports,17, 18 but this recommendation was followed only in Hungarian pregnant women with GH. Direct vasodilators, such as hydralazine and β-adrenergic receptor blockers are also effective and safe.17 Our previous study did not indicate a teratogenic and/or fetotoxic effect of calcium receptor antagonists.19 However, angiotensin converting enzyme inhibitors20 and angiotensin II receptor blockers/antagonists21 are contraindicated, but 10 pregnant women with GH were treated with captopril in our data set.
The strengths of the HCCSCA are that it is a population-based, large data set including 1579 and 1098 pregnant women with prospectively and medically recorded CH and GH in an ethnically homogeneous Hungarian (Caucasian) population. Additional strengths are the medically recorded pregnancy complications and birth outcomes, in addition to the available data of potential confounders.
However, this data set also has limitations: (i) The severity of CH and GH is determined by the medical treatment. (ii) Other pregnancy outcomes, for example, miscarriages, are not known. (iii) We were not able to evaluate the recently introduced drugs for the treatment of hypertension. (iv) Lifestyle factors cannot be evaluated in the total data set because of the unreliability of maternal self-reported data.22 In this study, there was a somewhat higher percentage of smokers among pregnant women affected with CH than in pregnant women without CH.
Previously, the opinion of experts was that hypertension without preeclampsia had no adverse effect on the fetus,23 but mainly fetal and perinatal death were evaluated. The above statement has been confirmed in untreated, that is, mild, CH in Hungarian pregnant women. However, intrauterine growth retardation is found more frequently in newborns of hypertensive women, along with an increase of frequency and severity of maternal blood pressure.24 This finding was confirmed in our study because a 2.2-fold higher risk for low-birthweight newborns was found. In addition, a strong association between severe CH and a higher risk for some pregnancy complications, including threatened abortions, preterm deliveries and placental disorders, was clear in our data set. These pregnancy complications and adverse birth outcomes may have a causal association with placental dysfunction as a common denominator. It is possible that these pregnant women had such severe CH that antihypertensive treatment was not able to neutralize its harm, that the antihypertensive treatments were not appropriate and/or effective, or, perhaps, that related drug treatments contributed to these adverse effects.25, 26, 27, 28
In general, monotherapy is preferred over polytherapy (for example, in epileptic pregnant women). However, polytherapy seems to be more effective in patients with hypertension.29, 30, 31 Therefore, it would be necessary to introduce a more effective drug combination in the treatment of pregnant women with severe CH.
In conclusion, a higher risk was found for some pregnancy complications (threatened abortions, preterm deliveries, and placental disorders) and adverse birth outcomes (low birthweight and preterm birth) in pregnant women with treated CH, indicating antihypertensive treatment was not able to neutralize the harm of severe CH in pregnant women. Treated GH associated with a higher risk for gestational diabetes and low birthweight newborns. However, pregnant women with untreated CH and GH had no higher risk of pregnancy complications or adverse pregnancy outcomes.
References
Rashidi A, Rachman M, Wright JT . Diagnosis and treatment of hypertension. In: Fuster V, Walsh RA, O’Rourke RA, Poole-Wilson P (eds). Hurst's The Heart, 12th edn. McGraw Hill Medical: New York, NY, 2008, pp 1610–1629.
Rey E, LeLorier J, Burgess E, Lange IR, Leduc L . Report of the Canadian Hypertension Society Consensus Conference. 3. Pharmacologic treatment of hypertensive treatment in pregnancy. Can Med Assoc J 1997; 157: 1245–1254.
Churchill D . The new American guidelines on the hypertensive disorders of pregnancy. J Hum Hypertens 2001; 15: 583–585.
Gifford R, August P, Cunningham G, Green LA, Lindheimer MD, McNellis D, Roberts JM, Sibai BM, Taler SJ . Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol 2000; 183: S1–S15.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572.
Czeizel AE, Rockenbauer M, Siffel Cs, Varga E . Description and mission evaluation of the Hungarian Case-Control Surveillance of Congenital Abnormalities, 1980–1996. Teratology 2001; 63: 176–185.
Czeizel AE . The first 25 years of the Hungarian Congenital Abnormality Registry. Teratology 1997; 55: 299–305.
Czeizel AE, Petik D, Vargha P . Validation studies of drug exposures in pregnant women. Pharmacoepidemiol Drug Saf 2003; 2: 409–416.
Czeizel AE, Vargha P . Periconceptional folic acid/multivitamin supplementation and twin pregnancy. Am J Obstet Gynecol 2004; 191: 790–794.
Hall JE, Granger JP, Hall ME, Jones DW . Pathophysiology of hypertension. In: Fuster V, Walsh RA, O’Rourke RA, Poole-Wilson P (eds). Hurst's The Heart, 12th edn. McGraw Hill Medical: New York, NY, 2008, pp 1570–1609.
Pickering TG, Ogedegbe G . Epidemiology of hypertension. In: Fuster V, Walsh RA, O’Rourke RA, Poole-Wilson P (eds). Hurst's The Heart, 12th edn. McGraw Hill Medical: New York, NY, 2008, pp 1551–1569.
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ . Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev, update, CD002252.
NHBPEWG. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol 2000; 183: S1–S22.
Puho E, Métneki J, Czeizel AE . Maternal employment status and isolated orofacial clefts in Hungary. Cent Eur J Public Health 2005; 13: 144–148.
Massaro EJ, Rogers JM (eds). Folate and Human Development. Humana Press: Totawa, NJ, 2002.
Arias F, Zamora J . Antihypertensive treatment and pregnancy outcome in patients with mild chronic hypertension. Obstet Gynecol 1979; 53: 489–492.
Shepard TH, Lemire RJ . Catalog of Teratogenic Agents, 11th edn. Johns Hopkins University Press: Baltimore, 2004.
Ounsted M, Cockburn J, Moar VA, Redman CWl . Maternal hypertension with superimposed preeclampsia: Effects on child development at 7½ years. Br J Obstet Gynecol 1983; 90: 644–650.
Sorensen HT, Czeizel AE, Rckenbauer M, Steffensen FH, Olsen J . The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand 2001; 80: 379–401.
Hanssens M, Keirse MJ, Vankelecom F, Van Assche FA . Fetal and neonatal effects of treatment with angiotensin converting enzyme inhibitors in pregnancy. Obstet Gynecol 1991; 78: 128–135.
Lambot MA, Vermeylen D, Noel JC . Angiotensin-II-receptor inhibitors in pregnancy. Lancet 2001; 357: 1619–1620.
Czeizel AE, Petik D, Vargha P . Smoking and alcohol drinking during pregnancy. The reliability of retrospective maternal self-reported information. Cent Eur J Public Health 2004; 12: 179–183.
Rey E, Couturier A . The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol 1994; 171: 410–415.
Tervila L, Goecke C, Timonen S . Estimation of gestosis of pregnancy (EPH-gestosis). Acta Obstet Gynecol Scand 1973; 52: 235–241.
Sibai BM, Mabie WC, Shamsa R, Villar MA, Anderson GD . A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol 1990; 162: 960–967.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD, Klebanoff M, Vandorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G, National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol 2002; 186: 66–70.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, VanDorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G . Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1998; 338: 701–706.
Imperiale TF, Petrulis AS . A meta-analysis of low-dose aspirin for the prevention of pregnancy-induced hypertensive disease. JAMA 1991; 266: 461–466.
Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ, ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359: 2417–2428.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL, The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guideline for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). Eur Heart J 2007; 28: 1462–1536.
Chobanian AV . Does it matter how hypertension is controlled? N Engl J Med 2008; 359: 2485–2488.
Acknowledgements
This study was partly sponsored by a generous grant from Richter Gedeon Pharmaceuticals Ltd, Budapest, Hungary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bánhidy, F., Ács, N., Puhó, E. et al. The efficacy of antihypertensive treatment in pregnant women with chronic and gestational hypertension: a population-based study. Hypertens Res 33, 460–466 (2010). https://doi.org/10.1038/hr.2010.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.17
Keywords
This article is cited by
-
Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement
Hypertension Research (2022)
-
Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: a population-based study
Hypertension Research (2011)