Abstract
The principal means for reducing proteinuria in patients with chronic kidney disease are strong blockade of the renin–angiotensin system and strict regulation of blood pressure (BP). This study compared the efficacy of the maximum permissible doses of two common angiotensin receptor blockers (ARBs), namely valsartan (maximum dose=160 mg per day) and olmesartan (maximum dose=40 mg per day). We also investigated whether a high-dose ARB or the combination of an angiotensin-converting enzyme inhibitor with a high-dose ARB would be more renal protective. We recruited 87 poorly controlled hypertensive patients. In the first study, 50 patients without proteinuria were switched from valsartan (160 mg per day) to olmesartan (40 mg per day) for 4 months. In the second study, 37 patients with proteinuria were randomized to either switch from valsartan 160 mg per day to 40 mg per day olmesartan (n=19; Olm-G) or addition of 2.5–10 mg per day imidapril (stepped up by 2.5 mg per month) to valsartan at 160 mg per day (n=18; Imi-G). After 4 months, the BP level decreased (first study) from 157/88 mm Hg to 145/82 mm Hg (P<0.001) and (second study) from 149/86 mm Hg to 135/77 mm Hg and 145/82 mm Hg for Olm-G and Imi-G, respectively. Furthermore, in the second study, urinary protein/creatinine excretion was reduced from 2.0±1.8 g g−1 to 0.8±0.8 g g−1 (P=0.0242) in Olm-G and from 1.4±1.3 g g−1 to 0.9±1.0 g g−1 (P=0.0398) in Imi-G. The significance persisted after adjustment for BP or other risk factors. Our results suggested that the maximum dose of olmesartan was more effective than that of valsartan and comparable with the combination of valsartan and imidapril for reducing BP and proteinuria in poorly controlled hypertensive patients.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) has received considerable attention in the management of hypertension,1 and it is an independent risk factor for cardiovascular disease in patients with hypertension.2 Proteinuria is one of the clinical parameters for diagnosing renal damage, particularly glomerular hypertension, and it is a risk factor and predictor for cardiovascular events.3 Reducing glomerular pressure is a principal strategy for reducing proteinuria in patients with hypertension.4 To reduce glomerular pressure, blood pressure (BP) must be decreased, typically by reducing arteriolar resistance in the efferent renal arterioles.5, 6 Angiotensin II type 1 receptors are localized to both afferent and efferent renal arterioles.5, 6 Several multicenter randomized clinical trials have shown that both angiotensin II receptor blockers (ARBs)7 and angiotensin-converting enzyme inhibitors (ACEIs)8 can reduce proteinuria. On the basis of those results, ARBs and ACEIs are the drugs of choice for managing hypertensive patients with CKD, according to JSH2009 (Japanese Society of Hypertension Guidelines for the Management of Hypertension, 2009). The recommended BP for patients with CKD is lower than that for older patients with hypertension alone with no complications.
We hypothesized that patients with hypertension and CKD would benefit from treatment with an ARB that sufficiently reduced BP, on the basis of previous results obtained from meta-analyses and the IRMA2 multicenter clinical trial.9 However, the initial, standard and maximum doses for ARBs are under government regulation in Japan. We previously reported that the administration of valsartan at 160 mg per day was more effective for reducing BP and proteinuria than the administration of candesartan at 12 mg per day in patients with hypertension.10 The Val-HeFT11 and VALIANT12 trials demonstrated that 320 mg per day of valsartan had beneficial effects on the prognoses for chronic heart failure and ischemic heart disease. However, in Japan, the permitted maximum doses of candesartan and valsartan are lower than the doses used in those clinical trials. No studies have compared the BP-lowering and renal-protective effects of the permitted maximum doses of ARBs. Head-to-head comparisons of olmesartan with each of the other ARBs, administered at the label-recommended doses, showed that olmesartan was most effective at decreasing BP.13 This study focused on hypertensive patients who had not achieved optimal BP after at least 3 months of treatment with the maximum permitted valsartan dose. We had two aims: (1) to determine whether BP would be better controlled by switching from valsartan to the maximum permitted olmesartan dose; and (2) to determine, in patients with hypertension and CKD, whether BP and urinary protein excretion (UPE) would be better controlled by switching from valsartan to the maximum permitted olmesartan dose or by adding an ACEI to valsartan. Thus, we called this study the high-dose ARB with ACEI study (Hawaii) study.
Methods
Study population
We recruited 90 hypertensive outpatients of the Osaka University Hospital who had received 160 mg valsartan per day for at least 3 months. At the onset of this study, patients had not achieved optimal BP recommended by the JSH2009. According to the JSH2009, optimal BP was <130/80 mm Hg for managing hypertensive patients with diabetes or CKD; <130/85 mm Hg for patients <65 years of age without major complications; <140/90 mm Hg for patients >65 years of age; and <125/75 mm Hg for patients with proteinuria or >1.0 g per day UPE. Patients were excluded from the study when they had experienced a stroke or cardiovascular event during the previous year; had grade 2 or higher congestive heart failure, according to the New York Heart Association scale; had >3.0 mg per 100 ml serum creatinine; and/or had a history of dry cough when taking ACEIs. The use of other antihypertensive drugs was permitted, except ACEIs. The doses of concurrent drugs remained unchanged throughout the study. The protocol for this study was approved by the hospital ethics committee, and informed consent was obtained from all patients before switching from valsartan to olmesartan or before adding imidapril.
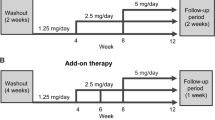
Study protocol for study 1
A total of 53 patients without CKD who had been taking 160 mg per day valsartan were recruited for study 1. Two patients were excluded because their average BP level was lower than optimal on the day of switching to olmesartan. Thus, 51 hypertensive patients were switched to 40 mg per day olmesartan. One month before the switch, we measured the cholesterol profile, uric acid, fasting blood glucose, as well as renal and liver functions. BP and pulse rate (PR) were evaluated 1 month before, the day of and 1 and 2 months after the switch. One patient left the study because of unpleasant side effects; thus, a total of 50 hypertensive patients were analyzed.
Study protocol for study 2
A total of 37 patients without CKD who had been taking 160 mg per day valsartan were recruited for study 2. All patients were randomly classified into two groups: 19 patients were switched to 40 mg per day olmesartan (Olm-G) and 18 patients had imidapril added to their valsartan regimen (Imi-G). Imidapril was gradually increased, from 2.5 mg per day for the first month to 5 mg per day for the second month, 7.5 mg per day for the third month and 10 mg per day for the fourth month. All other antihypertensive drugs were not modified. On the first day of the switch, we measured the cholesterol profile, uric acid, fasting blood glucose and liver function. Every month after initiation, we evaluated BP, PR, serum creatinine and UPE. All patients participated throughout the study.
BP and renal function measurements
Two BP and PR measurements were taken while the patient was seated and had rested for 10 min. BP was measured using BP-103iII (Nippon Colin, Tokyo, Japan). Averages of the two BP and PR measurements were automatically calculated, recorded and used in subsequent analyses. On the mornings of these hospital visits, subjects refrained from taking valsartan, olmesartan and imidapril.
We calculated the UPE adjusted for urinary creatinine. We estimated the glomerular filtration rate with a modified modification of diet in renal disease equation: glomerular filtration rate (ml min−1 per 1.73 m2)=175 × (serum creatinine)−1.154 × (age)−0.203 × 0.741 ( × 0.742 for females).14
Statistical analysis
Data were analyzed using commercially available statistical software (JMP version 5.1.1; SAS Institute, Cary, NC, USA). Differences in responses to treatment with olmesartan and valsartan were assessed with paired t-tests. Differences in low and high UPEs were assessed by one-factor ANOVA (analysis of variance) and Fisher's test. We used multiple regression analysis to evaluate influential factors for reducing UPE. P-values <0.05 were considered statistically significant.
Results
Results are expressed as mean±s.d.
Patient characteristics
Table 1 summarizes the characteristics of the 87 patients who completed studies 1 and 2. In study 2, the frequency of male patients was high (86%) and the mean age was slightly low compared with patients in study 1; however, these were not different between the Olm-G and Imi-G groups. Before entering this study, 68 participants had been taking anti-hypertensive drugs in addition to valsartan at 160 mg per day. These included calcium channel blockers (n=62, 71%), β-blockers (n=27, 31%), diuretics (n=21, 24%) and α-blockers (n=6, 7%). These ‘other hypertensive drugs’ were continued without change throughout the study. The mean number of concurrent anti-hypertensive drugs taken per patient was 2.3±1.0. Common serum factors that indicate liver function (Table 1) did not change during the study. There were no significant differences between the Olm-G and Imi-G groups.
BP and PR changes in study 1
Both BP and PR measurements were comparable 1 month before and on the day of switching from valsartan to olmesartan (Table 2). However, after the switch to olmesartan, systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean BP (MBP) levels were significantly lower (+1 month, +2 months; Table 2). After switching to olmesartan, the mean (averaged +1 and +2 month values) SBP was 144.7±13.8 mm Hg and the mean DBP was 80.9±10.1 mm Hg. These were significantly reduced compared with the mean values (averaged 1 month before and day of switch values) before the switch (SBP, 154.7±10.0 mm Hg, P<0.0001; DBP, 86.9±8.0 mm Hg, P<0.0001). In contrast, after the switch, the mean PR (73.9±13.3 b.p.m.) was not different than the mean before the switch (PR, 72.9±13.7 b.p.m., P=0.2391). A frequency analysis showed that after switching to olmesartan, 7 (14%) patients had elevations in SBP, 25 (50%) had <10 mm Hg reductions in SBP, 11 (22%) had 10–20 mm Hg reductions in SBP and 7 (14%) had >20 mm Hg reductions in SBP (data not shown).
BP in study 2
The results of study 2 are shown in Table 3. In the Olm-G group, SBP was significantly reduced after 1 month (from 149±19 mm Hg to 142±20 mm Hg; P<0.05), and finally reached 135±15 mm Hg after 4 months (P<0.01 vs. 0 month). In the Imi-G group, SBP was not significantly reduced after the first month (from 145±17 mm Hg to 140±20 mm Hg), but finally reached 138±15 mm Hg after 4 months (P<0.05 vs. 0 month). The MBP value is also an important parameter for managing CKD; therefore, we also evaluated MBP in study 2. In the Olm-G group, MBP was significantly reduced at 1 month (from 107±15 mm Hg to 102±16 mm Hg; P<0.01), 2 months (102±14 mm Hg; P<0.05 vs. 0 month), 3 months (101±15 mm Hg; P<0.05 vs. 0 month) and finally reached 97±14 mm Hg at 4 months (P<0.01 vs. 0 month). In the Imi-G group, MBP was not significantly reduced for the first 1–3 months, but finally reached 100±11 mm Hg at 4 months (P<0.05 vs. 0 month).
Influences of UPE in study 2
We evaluated renal function by analyzing UPE and the estimated glomerular filtration rate. The baseline (0 month) UPE was not statistically different between the groups. In the Olm-G group, UPE was significantly reduced after 1 month (from 2.0±1.8 g g−1 to 1.3±1.2 g g−1 creatinine; P<0.05), and finally reached 0.8±0.8 g g−1 creatinine after 4 months (P<0.05 vs. 0 month). In the Imi-G group, UPE was not significantly reduced after 1 month (from 1.4±1.3 g g−1 to 1.1±1.0 g g−1 creatinine), but finally reached 0.9±1.0 g g−1 creatinine after 4 months (P<0.05 vs. 0 month). However, there were no significant changes in the estimated glomerular filtration rate during follow-up in either group.
UPE reduction and other factors
To clarify the influence of other factors on UPE reduction, we used a multiple regression analysis to examine correlations between the fractional UPE reduction, defined as (UPE at 0 month−UPE at 4 months)/UPE at 0 month and other variables (Table 4). This showed that no other factors influenced the observed UPE reductions.
Discussion
In this study, we first demonstrated in hypertensive patients without CKD that 40 mg per day olmesartan resulted in an enhanced reduction of SBP (10.1 mm Hg) and DBP (6.0 mm Hg) compared with 160 mg per day valsartan. A previous meta-analysis showed that several ARBs, such as losartan, irbesartan, candesartan, valsartan and olmesartan, had a placebo-corrected dose effect on BP. In that report, at the recommended maintenance dose (80 mg per day), the net effect of valsartan on SBP and DBP was very similar to that observed with olmesartan at 20 mg per day. However, at twice the recommended maintenance dose (160 mg per day), the net effect of valsartan on SBP and DBP appeared to be lower than that observed with olmesartan at 40 mg per day. On the basis of that evidence, we postulated that the BP-lowering effect of 40 mg olmesartan would be greater than that of 160 mg valsartan. However, there was no direct head-to-head study that showed an advantage of olmesartan over valsartan. Several current guidelines for managing essential hypertension15, 16 and many large, multicenter trials17, 18 have suggested that strict reduction of BP is the most important factor for preventing cardiovascular mortality and morbidity in hypertensive patients. The patients included in this study were hypertensive and could not achieve optimal BP with antihypertensive treatments, including 160 mg per day valsartan. We found that >80% of the study participants had reduced SBP after switching from 160 mg per day valsartan to 40 mg per day olmesartan.
We also found that 40 mg per day olmesartan more effectively reduced UPE than did 160 mg per day valsartan. Furthermore, olmesartan was as effective as the combination of valsartan 160 mg per day and imidapril 10 mg per day for reducing UPE after 4 months. These results were consistent with those from the modification of diet in renal disease study, which showed that the reduction in MBP was useful for decreasing UPE.19 Other clinical data suggested that high doses of ARBs reduced UPE more effectively than did low doses; examples include 96 mg per day candesartan,20 640 mg per day valsartan21 and 300 mg of irbesartan.9 Thus, the UPE reduction observed in this study was not a specific effect of olmesartan, but of high doses of ARBs, in general.
There is no statistical difference in UPE and BP at baseline; however, UPE and BP in the Olm-G group showed higher tendency than those in the Imi-G group. To clarify factors that influenced the reduction of UPE, we also performed multiple regression analysis in Table 4. We did not find influenced factors, including BP and UPE at baseline, BP reduction and medication, such as switching to olmesartan or adding imidapril. According to these evidences, olmesartan 40 mg per day and valsartan 160 mg per day with imidapril 10 mg per day equally reduced UPE in patients with hypertension and CKD.
This study demonstrated that the maximum permitted dose of olmesartan produced a greater reduction in BP and UPE than did the maximum allowable dose of valsartan. Moreover, high-dose olmesartan reduced UPE with an efficacy comparable with that achieved with high-dose valsartan combined with imidapril. These results do not discount the efficacy of valsartan in preventing cardiovascular events and total mortality in many multicenter trials.11, 12, 18 Our results suggested that a high dose of olmesartan is beneficial for managing hypertensive patients, especially those with impaired renal function.
Study limitations
This study had several limitations. The most important limitation was the protocol; only one switch was performed to compare two anti-hypertensive drugs in study 1. A crossover or randomized study would be necessary to properly compare olmesartan and valsartan treatments. From an ethical standpoint, it was not advisable to switch patients back to 160 mg valsartan after they had achieved optimal BP with 40 mg olmesartan. In study 2, although we randomized the participants into olmesartan and imidapril groups, BP and UPE in the olmesartan group were slightly higher, although the difference was not statistically significant. A study with a larger number of participants will be required to confirm our results. In this study, subjects were preferentially selected to create a group whose hypertension was poorly controlled with valsartan treatment. Thus, it was not surprising that the study patients did not respond well to valsartan.
References
Peralta CA, Kurella M, Lo JC, Chertow GM . The metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens 2006; 15: 361–365.
Segura J, Campo C, Gil P, Roldan C, Vigil L, Rodicio JL, Ruilope LM . Development of chronic kidney disease and cardiovascular prognosis in essential hypertensive patients. J Am Soc Nephrol 2004; 15: 1616–1622.
De Leeuw PW, Thijs L, Birkenhager WH, Voyaki SM, Efstratopoulos AD, Fagard RH, Leonetti G, Nachev C, Petrie JC, Rodicio JL, Rosenfeld JJ, Sarti C, Staessen JA . Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst-Eur trial. J Am Soc Nephrol 2002; 13: 2213–2222.
Marin R, Gorostidi M, Fernandez-Vega F, Alvarez-Navascues R . Systemic and glomerular hypertension and progression of chronic renal disease: the dilemma of nephrosclerosis. Kidney Int 2005; 58: S52–S56.
Hayashi K, Wakino S, Homma K, Sugano N, Saruta T . Pathophysiological significance of T-type Ca2+ channels: role of T-type Ca2+ channels in renal microcirculation. J Pharmacol Sci 2005; 99: 221–227.
Wang T, Takabatake T . Effects of vasopeptidase inhibition on renal function and tubuloglomerular feedback in spontaneously hypertensive rats. Hypertens Res 2005; 28: 611–618.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869.
Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G . Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005; 365: 939–946.
Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878.
Ohishi M, Takagi T, Ito N, Tatara Y, Hayashi N, Shiota A, Iwamoto Y, Katsuya T, Rakugi H, Ogihara T . Renal protective effect in hypertensive patients: the high doses of angiotensin II receptor blocker (HARB) study. Hypertens Res 2007; 30: 1187–1192.
Cohn JN, Tognoni G . A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675.
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM, Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM . Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906.
Zannad F, Fay R . Blood pressure-lowering efficacy of olmesartan relative to other angiotensin II receptor antagonists: an overview of randomized controlled studies. Fundam Clin Pharmacol 2007; 21: 181–190.
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Hirakata H, Watanabe T, Moriyama T, Ando Y, Inaguma D, Narita I, Iso H, Wakai K, Yasuda Y, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S . Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol 2007; 11: 41–50.
European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology Guidelines for the Management of Arterial Hypertension. J Hypertens 2003; 21: 1011–1053.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ . The Seventh Report of the Joint National Committee On Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003; 289: 2560–2572.
ALLHAT Officers Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–2997.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363: 2022–2031.
Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL . Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995; 123: 754–762.
Weinberg M, Weinberg A, Cord R, Zappe D . The effect of high-dose angiotensin II receptor blockade beyond maximal recommended doses in reducing urinary protein excretion. JRAAS 2001; 2 (suppl 1): S196–S198.
Hollenberg NK, Parving HH, Viberti G, Remuzzi G . The Diovan reduction of proteinuiria (drop) study: albuminuria response to high-doses of valsartan in type 2 diabetes mellitus. Circulation 2006; 114: II–61.
Acknowledgements
We are grateful to Ms Kazuko Iwasa and Ms Eriko Nagata for their secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohishi, M., Takeya, Y., Tatara, Y. et al. Strong suppression of the renin–angiotensin system has a renal-protective effect in hypertensive patients: High-dose ARB with ACE inhibitor (Hawaii) study. Hypertens Res 33, 1150–1154 (2010). https://doi.org/10.1038/hr.2010.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.145
Keywords
This article is cited by
-
A Comparative Effectiveness Study of Renal Parameters Between Imidapril and Amlodipine in Patients with Hypertension: A Retrospective Cohort Study
Cardiology and Therapy (2017)
-
Antihypertensive Efficacy of Olmesartan Medoxomil and Ramipril in Elderly Patients with Mild to Moderate Hypertension Grouped According to Renal Function Status
High Blood Pressure & Cardiovascular Prevention (2012)
-
The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin–angiotensin–aldosterone system, oxidative stress and endogenous digitalis in the brain
Hypertension Research (2011)