Abstract
The C-repeat binding factor (CBF) transcription factor is involved in responses to low temperature and water deficit in many plant species. Overexpression of CBF genes leads to enhanced freezing tolerance and growth inhibition in many species. The overexpression of a peach CBF (PpCBF1) gene in a transgenic line of own-rooted apple (Malus×domestica) M.26 rootstock (T166) trees was previously reported to have additional effects on the onset of dormancy and time of spring budbreak. In the current study, the commercial apple cultivar ‘Royal Gala’ (RG) was grafted onto either non-transgenic M.26 rootstocks (RG/M.26) or transgenic M.26 (T166) rootstocks (RG/T166) and field grown for 3 years. No PpCBF1 transcript was detected in the phloem or cambium of RG scions grafted on T166 rootstocks indicating that no graft transmission of transgene mRNA had occurred. In contrast to own-rooted T166 trees, no impact of PpCBF1 overexpression in T166 rootstocks was observed on the onset of dormancy, budbreak or non-acclimated leaf-cold hardiness in RG/T166 trees. Growth, however, as measured by stem caliper, current-year shoot extension and overall height, was reduced in RG/T166 trees compared with RG/M.26 trees. Although flowering was evident in both RG/T166 and RG/M.26 trees in the second season, the number of trees in flower, the number of shoots bearing flowers, and the number of flower clusters per shoot was significantly higher in RG/M.26 trees than RG/T166 trees in both the second and third year after planting. Elevated levels of RGL (DELLA) gene expression were observed in RG/T166 trees and T166 trees, which may play a role in the reduced growth observed in these tree types. A model is presented indicating how CBF overexpression in a rootstock might influence juvenility and flower abundance in a grafted scion.
Similar content being viewed by others
Introduction
C-repeat binding factor (CBF)/drought response element binding (DREB) proteins function as transcription factors that regulate plant responses to low temperature and water deficit. They represent a sub-family within the larger AP2-domain family of transcription factors. CBF genes have been identified in a wide range of herbaceous1–4 and woody plants.5–9 At least five CBF genes9 have been identified in both apple (Malus×domestica) and peach (Prunus persica) that exhibit a variety of patterns of expression in response to low temperatures.10–12
Overexpression of CBF genes has been demonstrated to increase freezing tolerance in several plant systems, including homologous and/or heterologous expression in Arabidopsis and tobacco,2 tomato,13 potato,14 poplar,6 grape,15 Eucalyptus8 and apple.10 Growth inhibition in both herbaceous16–19 and perennial, woody plants has also been observed, including Eucalyptus,8 grape15 and apple.10,12,20 Wisniewski et al. 10 also reported that apple trees (M.26 rootstock) overexpressing the peach (Prunus persica) PpCBF1 gene, in contrast to non-transformed plants, responded to short-day photoperiods by entering dormancy and exhibiting premature leaf senescence. The short-day-response was unexpected as apple has been shown to respond to low temperature rather than photoperiod cues for regulating the onset of dormancy.21 In a subsequent report, Artlip et al.20 noted that self-rooted, field-planted apple trees overexpressing PpCBF1 exhibited all the characteristics observed in growth chamber studies, including enhanced cold hardiness in acclimated and non-acclimated leaves, growth inhibition, early dormancy and premature leaf senescence. They also observed that spring budbreak was delayed in the transgenic trees and that the overall phenotype of the transgenic trees was stable over at least three seasons of growth.
Wisniewski et al.12 examined several genes (RGL, DAM and EBB) that had been reported to be correlated with growth and dormancy in other woody species and compared their level of expression with non-transformed trees. RGL (DELLA) genes are involved with gibberellic acid (GA) signaling, acting to suppress GA-mediated growth.22 Achard et al.23 reported that overexpression of CBF proteins resulted in decreased GA levels and increased RGL/DELLA protein levels, thus inhibiting growth. Wisniewski et al.12 showed that two of six apple RGL/DELLA genes exhibited temporally elevated expression levels over the course of a year in trees overexpressing PpCBF1 compared with non-transformed trees. Genes associated with dormancy in perennial, woody plants were also examined.12 Although the expression levels of three DAM (Dormancy-associated MADS-box) genes did not differ between transgenic and non-transgenic trees in bark tissues during most of the season, distinct differences were observed in buds just before and during budbreak. More specifically, two MdDAM genes exhibited higher expression levels in transgenic trees. Elevated levels of EBB1 (Early Budbreak 1) gene transcripts have been reported to be highly correlated with budbreak in poplar.24 Elevated expression levels of the apple homolog, MdEBB1, were also shown to correspond to budbreak in field-grown apple trees. Increases in MdEBB1 were temporally delayed in the PpCBF1 overexpressing trees, correlating well with the observed delay in budbreak in those trees.
Rootstocks are widely used in perennial fruit crops to convey a range of horticultural traits, with the main influence being on growth control or dwarfing. As the parent material that was used for the overexpression of PpCBF1 was an apple rootstock (M.26), a logical extension of the previous studies was to determine what physiological and morphological effects would be transmitted to any scions that were grafted on the transgenic rootstock. Although the impact of rootstocks on scions is in part regulated via hormones,25,26 graft transmission of mRNAs from transgenic, herbaceous donor plants to herbaceous-grafted plants has also been reported,27 with transmission of silencing RNAs (siRNAs) across the graft union recently reported in cherry.28 In addition, transmission of transgenic proteins across the graft union has also been reported in woody plants.29,30 In the current study, ‘Royal Gala’ was bud-grafted onto either non-transgenic M.26 or transgenic M.26 (T166) rootstocks (constitutively expressing a peach CBF, PpCBF1). Self-bud-grafted non-transgenic M.26 and T166 were also created along with own-rooted non-transgenic M.26 and T166 rootstocks. Six-month-old trees were planted and physiological, phenological and gene expression data acquired over 3 years.
Materials and methods
Plant material
The peach (Prunus persica) PpCBF1 gene was cloned and an overexpressing transgenic line, T166, was developed as reported in Wisniewski et al.10 Explants of non-transformed ‘Royal Gala’, non-transformed M.26 and transformed T166 were propagated in tissue culture as described by Norelli et al.31 and Ko et al.,32 with root induction as described by Bolar et al.33 After establishment, propagated trees were grown in an environmental chamber and greenhouse as described in Wisniewski et al.10 Buds of ‘Royal Gala’ were T-budded onto either M.26 or T166 trees. The grafted plants were grown for at least 6 months before being planted in the field. Own-rooted M.26 and T166 rootstocks were grown as controls per Artlip et al.20 M.26 and T166 buds were also self-T-budded on their respective parent trees in order to assess whether grafting altered phenotypical or phenological aspects compared with own-rooted trees.12,20
Field planting
Ten trees each of ‘Royal Gala’ on M.26 (RG/M.26) or T166 (RG/T166) rootstocks, and own-rooted M.26 and T166 trees were planted in October 2012. Ten own-budded M.26 and T166 trees were planted in October 2013. All plantings were located on the grounds of the USDA-ARS, Appalachian Fruit Research Station, Kearneysville, WV, USA. The planting design consisted of five rows, with two trees of each tree type, randomly assigned a planting location within each row. All the planted trees had been growing in a greenhouse for 6 months before the time of planting. Trees were not pruned in order to assess natural growth, however, conventional pesticide management practices were applied during the course of the study.
Growth and phenological measurements
Growth measurements were taken monthly during the growing season (March–November). Caliper (stem diameter) data were taken at a point 20 cm above the graft union. Overall height data were taken from the tip of the main stem to the ground, and current season’s shoot length was taken from the terminal bud scar of the previous year’s growth to the tip of the current season’s growth. Dates of budbreak were recorded during spring 2013 and 2014, and expressed as Julian Day of Year. In 2013, the date of budbreak of the terminal bud on the main shoot and 20 lateral buds was recorded. In 2014, the date of budbreak of the terminal bud and 20 lateral buds of the main shoot was recorded, as well as the terminal bud and 20 laterals buds on two lateral shoots. Budbreak data were recorded on three trees each of ‘Royal Gala’ grafted on either M.26 or T166 and for own-rooted trees.
Assessment of freezing tolerance by ion-leakage assays
Leaves from field-grown trees were harvested pre-dawn during late-June 2014, thus minimizing any impact of low temperature exposure on cold acclimation, leaf health, growth status or any interfering effect of variable leaf water status. Ion-leakage assays were performed as described in Artlip et al.20 The LT50 (lethal temperature at which 50% of the tissue is killed) was derived as described by Zhang and Willison.34 The percent leakage data from unfrozen controls were set at 0% and leakage obtained at the lowest test temperature was set at 100%, thus normalizing the data over a range of 0–100%. The data were plotted with Origin version 7.5 (OriginLab, Northampton, MA, USA) and a sigmoidal curve fitted to the plotted data. All measurements were replicated three times where a replicate represents an individual tree (n=9).
RT-qPCR
Bark tissue was harvested from lateral branches of RG/ M.26, RG/ T166 and own-rooted T166 trees taken during June, July and August 2015. These months were chosen based on the growth data of the current study and that of Artlip et al.,20 and expression data reported by Wisniewski et al.,12 who found that the growth-related MdDELLA (MdRGL) genes had variable kinetics during those months. Bark was scraped directly into liquid N2, lyophilized and stored at −20 °C until used. Total RNA was isolated from bark tissue samples using Concert Plant RNA Reagent (Invitrogen, Carlsbad, CA, USA), treated with DNase (Turbo DNA-free Kit; Ambion, Austin, TX, USA) and then diluted based on the preliminary testing for optimal response. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis was performed using appropriate quantities of total RNA (per preliminary testing) as a template with the Power SYBR Green RNA-to-Ct 1-Step Kit (Applied Biosystems, Foster City, CA, USA) and 2.0 pmol of each primer per reaction (Supplementary Table 1); no-RT control reactions were included to ensure no residual DNA contamination. A ViiA 7 Real-Time PCR System (Applied Biosystems) was set to cycle as follows: cDNA synthesis at 48.0 °C for 30 min; 95.0 °C denaturation for 10 min; 40 cycles of 95.0 °C for 15 s followed by 52.0–57.0 °C (depending on primers used) for 1 min; followed by ABI-specified hold and melt curve stages. Primers were verified for specificity by using genomic DNA template and assessing the resulting amplicon by agarose gel electrophoresis and qPCR with genomic DNA on the ViiA 7 Real-Time PCR System; all primers had a single band and single peak. Primer efficiency was also verified for all primer sets by constructing a standard curve using qPCR data. Three technical replicates were used for each of the three biological replicates (tree). The standard curve method (user bulletin no. 2; Applied Biosystems http://www3.appliedbiosystems.com/cms/groups/mcbsupport/documents/generaldocuments/cms_040980.pdf) was used to calculate transcript abundance relative to MdoLTL1 as a reference gene.35 Other endogenous reference genes were also examined, but MdoLTL1 was determined to be the best overall reference gene using NormFinder.36 To weight the importance of biological variation over technical variation, technical replicates were nested within biological replicates in calculating the mean square error term. The expression of PpCBF1 was assessed slightly differently. Rather than the final melt step in the RT-qPCR protocol previously described, reactions were separated on a 2% agarose gel, stained with ethidium bromide, and scanned on a Typhoon FLA 9500 scanner (GE Healthcare, Piscataway, NJ, USA) to determine if any and how much product was generated.
Results
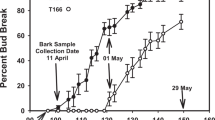
After establishing the planting in the fall of 2012, phenotypic measurements were commenced in the spring of 2013. Budbreak data, defined as the green tip stage in vegetative buds,37 is presented in Figure 1. No discernable impact of the T166 transgenic rootstock on the time of budbreak of ‘Royal Gala’ was observed. Budbreak in RG/T166 and RG/M.26 went from ~5 to 90% in a 24-h period in 2013 stimulated by the very warm temperatures (maximum temperatures⩾28 °C) that occurred for several days just before the abrupt rise in percent budbreak. The timing and rapid increase in percent budbreak was similar in 2014. This was in sharp contrast to the delayed budbreak observed in own-rooted and self-budded T166 trees.
Budbreak of RG/M.26, RG/T166 and T166 trees over two seasons. (a) 2013 data. (b) 2014 data. Twenty buds basipetal to the terminal bud of the central axis were monitored for budbreak (green tip) and percent budbreak calculated. Symbols represent the mean±s.e. (n=360). Black squares, RG/M.26; red circles, RG/T166; blue triangles, own-rooted T166.
Graft transmissibility of enhanced freezing tolerance from the transgenic T166 rootstock to the ‘Royal Gala’ scion was assessed on leaves in June 2014 by ion leakage. The LT50 (temperature at which 50% of the tissue is killed) in leaves of RG/M.26 versus RG/T166 were compared. Results indicated that there were no significant differences in the LT50 of leaves between the two types of trees (Figure 2). In contrast, leaves obtained from own-rooted T166 trees displayed a significant enhancement in freezing tolerance. No detectable level of PpCBF1 mRNA was observed in the phloem/cambium tissues of ‘Royal Gala’ bark tissues of scions that had been grafted on to T166 rootstocks, which implies that there was no graft transmittance of transgene mRNA from the rootstock to the grafted scion (Figure 3a). The expression of two endogenous apple CBF genes, MdCBF2 and MdCBF4, was also examined by RT-qPCR to determine whether or not PpCBF overexpression in the T166 rootstock influenced their expression in the RG scions (Figures 3b and c). In June, the highest level of expression of MdCBF2 was observed in RG/T166 and T166 trees while the lowest level was observed in RG/M.26 trees. Levels of MdCBF2 expression were similar in all three tree types in July and September. MdCBF4 expression was generally low and similar in all three tree types but exhibited a sharp increase in T166 trees in September.
Freezing tolerance of non-acclimated (June) leaves collected from RG/M.26, RG/T166 and T166 own-rooted trees. No difference in freezing tolerance was observed between RG/M.26 and RG/T166 leaves, while T166 exhibited enhanced freezing tolerance. Black squares, RG/M.26; red circles, RG/T166; blue triangles, own-rooted T166. Symbols represent mean±s.e. (n=3). Each biological replicate (tree) consisted of three technical replicates.
Expression of PpCBF1 and native MdCBF genes in scion bark tissues of RG/M.26, RG/ T166 and T166 own-rooted trees during the summer growing season. (a) PpCBF1 expression as determined by PCR. The PCR products were separated on a 2% agarose gel. (b) RT-qPCR of MdCBF2. (c) RT-qPCR of MdCBF4. Black squares, RG/M.26; red circles, RG/T166; blue triangles, own-rooted T166. Data represent the mean±s.e. (n=3). Each biological replicate (tree) was composed of three technical replicates.
Growth of RG/T166 trees and RG/M26 trees, as measured by stem diameter, current-year shoot extension and overall height, was recorded over a 3-year period (Figure 4). Increases in caliper (stem diameter) and overall height in RG/T166 trees were consistently less than in RG/M.26 trees in all three growing seasons (Figures 4a and b). Differences between the trees at the onset of the measurements reflect the status of trees after growing in the greenhouse for ~6 months. Current-year shoot growth in RG/ T166 trees was significantly less than in RG/M.26 in both 2013 and 2015 but not 2014 (Figure 4c). Field notes indicate, however, that several of the tagged branches used to measure current-year shoot growth in RG/M.26 trees exhibited death of a terminal bud. As the sub-terminal buds did not grow appreciably after the death of the terminal bud, this impacted the recorded overall current-year shoot growth in 2014. No obvious reason for the death of the terminal buds was evident. Representative photos illustrate the growth disparity between the tree types (Figure 5). Differences in the number of lateral branches present in the two tree types can also be observed in Figure 5. RG/ M.26 trees generally had a higher number of lateral branches than RG/ T166 trees.
Growth of RG/M.26 and RG/T166 trees over three growing seasons. (a) Caliper (stem diameter) 20 cm above the graft union. (b) Current-year shoot growth taken from previous season’s bud scar to the shoot terminus. (c) Overall height. Black squares, RG/ M.26; red circles, RG/ T166. Symbols represent mean±s.e. (n=5).
The RG/M.26 and RG/T166 trees began to flower during the second growing season in the field, 2014 (Figure 6). The number of trees with bloom clusters and the average number of bloom clusters per tree differed markedly between the RG/M.26 and RG/ T166 trees in 2014 and subsequently in 2015. RG/M.26 trees consistently exhibited greater levels of flowering than RG/T166 trees (Table 1). Earlier research reported that overexpression of PpCBF1 resulted in both reduced growth and the overexpression of two RGL (DELLA) genes, MdRGL3a and MdRGL3b.12 In general, DELLA genes have been associated with a suppression in GA responses. In the current study, the expression of four endogenous RGL (DELLA) genes was examined during June, July and September 2015, the time period of very active growth (Figure 4). Although RGL expression levels varied over this time period, levels of MdRGL 1a, 1b and 3a expression in RG/T166 and own-rooted T166 were similar in June and contrasted with the level of RGL expression in RG/M.26 trees (Figure 7). Subsequently, the pattern of RGL expression in July and September was similar in all three tree types. In general, RGL expression decreased in all three tree types over the measured time course. The only exception to the stated patterns was for MDRGL3b when levels of expression were significantly higher in RG/M.26 trees than in the other two tree types (T166 and RG/T166).
Photo of representative of RG/M.26 and RG/T166 trees in the spring of 2014 illustrating differences in the level of flowering in the two tree types. A greater number of RG/M.26 trees had floral bud clusters than RG/T166 trees. (a) Representative RG/M.26 tree. (b and c) Close up photos of a RG/M.26 tree showing numerous floral bud clusters. (d) Representative RG/T166 tree. (e and f) Close up photos of a RG/T166 tree showing no floral bud clusters.
RT-qPCR analysis of MdRGL gene expression in bark tissues of scions from RG/M.26, RG/T166 and T166 own-rooted trees during the summer growing season. (a) MdRGL1a. (b) MdRGL1b. (c) MdRGL3a. (d) MdRGL3b. Black squares, ‘RG’/M.26; red circles, ‘RG’/T166; blue triangles, own-rooted T166. Symbols represent mean±s.e. (n=3). Each biological replicate (tree) was composed of three technical replicates.
Discussion
Previous studies of overexpression of a peach CBF gene (PpCB1) in M.26 apple rootstocks revealed a significant impact on several developmental and phenological parameters.10,12,20 Transgenic trees (line T166) exhibited increased levels of non-acclimated and acclimated freezing tolerance, response to short photoperiod leading to the early onset of dormancy and early leaf senescence, and delayed budbreak, relative to non-transgenic M.26 trees. A reduction in overall height, stem caliper and current-year shoot growth was also observed in T166 trees. This was true in both greenhouse and several years of field studies.10,12,20 As rootstocks are typically used to control growth and impart other horticultural traits in scion varieties,26 the purpose of the present study was to determine if the physiological traits observed in T166 trees could be imparted to or at least influence scions that were grafted on to T166 rootstocks.
In previous studies and the current study, own-rooted T166 trees overexpressing the PpCBF1 gene exhibited early dormancy, early leaf senescence and delayed spring budbreak compared with non-transgenic M.26 trees.10,12,20 These physiological and phenological attributes, however, were not observed in the scion (Royal Gala) that was grafted on the T166 rootstock. No major differences were observed between RG/T166 and RG/M.26 trees in any of these parameters. In addition, no differences were observed in non-acclimated leaf-freezing tolerance between RG/T166 and RG/M.26 trees. These results clearly indicate that it would not be possible to use this transgenic rootstock to convey the dormancy and freezing tolerance attributes exhibited by the transgenic rootstock to different scion genotypes grafted on to this rootstock. Transmission of mRNA from herbaceous transgenic donor plants to recipient plants through a graft union via the phloem have been documented.27 No evidence of PpCBF1 mRNA, however, was observed in the ‘Royal Gala’ scions that had been grafted on T166 transgenic rootstocks (Figure 3a). Although elevated levels of endogeneous MdCBF2 were present in the RG/T166 trees in June, relative to both RG/M.26 trees and T166 trees, when freezing tolerance was assayed, no impact on freezing tolerance was observed in the RG/T166 trees (Figure 3b). In contrast, MdCBF4 levels were greatly elevated in September only in the T166 trees (Figure 3c). These data suggest that MdCBF2 is perhaps more related to water deficit and responsible for the different levels of expression observed between the tree types. In contrast, MdCBF4 may be more related to freezing tolerance, thus becoming greatly elevated in the T166 September samples due to fact that the T166 trees had entered dormancy and responded to a shortening photoperiod while the other tree types (RG/M.26 and RG/T166) had not. In this regard, leaves serve as the point of perception of photoperiod.38 In the present study, transgenic rootstock leaves were not present on the RG/T166 trees. This could account for the lack of impact of the rootstock on the various dormancy attributes in the scion in RG/T166 trees. Without transgenic leaves present, the rootstock could not respond to the shortening photoperiod and thus impact dormancy the way it does in own-rooted T166 trees. Trees possessing branches and leaves derived from both scion bud grafts and the T166 transgenic rootstock are presently being developed to test this hypothesis.
Despite the lack of impact of the T166 transgenic rootstock on scion dormancy and freezing tolerance, a consistent and significant effect was observed on scion growth and precocity, as evidenced by reduced growth and flowering in RG/T166 trees (Figures 4, 5, 6 and Table 1). Stem caliper, current-year shoot growth (except for 2014), and overall tree height was also reduced in RG/T166 trees relative to RG/M.26 trees (Figure 4). In addition, beginning in 2014, the number of trees with flowers was greater in RG/M.26 trees than it was in RG/T166 trees, as was the number of branches with flowers, and the total number of flower clusters.
Constitutive overexpression of CBF genes has been reported to result in diminished growth in several plant systems. Herbaceous plants such as Arabidopsis,17–19,39 potato14 and tomato13 have exhibited this phenotype when native or exogenous CBF genes were overexpressed. Woody plants, such as Eucalyptus,8 birch,5 poplar,6 grape15 and apple10,12,20 have also exhibited reduced growth with CBF gene overexpression. The reports in apple demonstrated that the reduced growth occurs not only in greenhouse and environmental chamber studies but over the course of several years when trees are planted in the field.10,12,20 Growth reduction on the order of 20% (stem diameter) and 13% (tree height) was seen in the current study in RG/T166 trees after three growing seasons, compared with RG/M.26 trees (Figure 4). Growth reduction was even more pronounced in own-rooted or own-grafted T166 trees, being reduced by 25% in stem diameter and 33% in overall height) after three growing seasons, compared with own-rooted M.26 trees (Supplementary Figure S1).
The disparity between RG/ T166 and RG/ M.26 trees extended beyond overall height and stem diameter as RG/ T166 trees also produced fewer lateral branches (Figure 5). Artlip et al.20 and Wisniewski et al.12 noted a similar trend when comparing field-grown, own-rooted T166 with M.26 trees.
Rootstocks are known to affect various scion traits, such as growth and tree architecture.40 The question arises, however, as to how the overexpression of a CBF gene can further the impact of a specific rootstock on the horticultural traits of a grafted scion cultivar. In regards to growth, Wisniewski et al.12 reported on the impact of CBF on the expression of RGL (DELLA) genes, which are generally considered to inhibit GA-mediated growth processes.41 They were able to demonstrate higher levels of expression of at least two native apple RGL genes and suggested that the high levels of these genes resulted in the inhibition of GA-mediated growth at the time of year when the rate of growth was greatest. Higher expression levels of three native RGL genes (MdRGL1a, 1b and 3a) were observed in the present study in RG/T166 and own-rooted T166 trees, compared with RG/M.26 trees. These high levels were observed in the month of June when growth rates were high. Overexpression of CBFs have been shown to decrease GA levels and increases DELLA levels in other plant systems.23,42,43 Moreover, Xu et al.44 demonstrated that RGL mRNA is phloem-mobile and that both acropetal and basipetal translocation of mRNAs of two GAI (=RGL=DELLA) genes can occur across the graft union of ‘Fuji’/Malus xiaojinensis trees. The combination of CBF effects on bio-active GAs, DELLAs and the reports on translocation across graft unions provide a framework to explain the observations reported in the current study.
In addition to the influencing scion growth and architecture, rootstocks are also known to effect time to flowering or precocity.40 In the present study, time to flowering was delayed in RG/T166 trees compared with RG/M.26 trees (Table 1). Although flowering commenced in RG/M.26 trees in the second season of growth (2014), flowering was very sparse in RG/T166 trees. This difference persisted in subsequent seasons with RG/M.26 trees exhibiting a greater number of trees with flowers, more branches with flowers on individual trees, and more flower clusters per flowering shoot (Figure 6; Table 1). The influence of the T166 rootstock on precocity and number of floral bud clusters is perhaps unexpected, given that dwarfing rootstocks tend to result in earlier bearing than semi-dwarfing or standard rootstocks,26 and T166 is clearly more dwarfing than M.26. However, Gilmour et al.40,41 also reported that overexpression of native CBF genes in Arabidopsis inhibited flowering. Therefore, the relationship between CBF overexpression and inhibition of flowering is not unprecedented. Nonetheless, the observations in the present study are in contrast to the general impact of dwarfing on flowering. Watson et al.40 in a study on the effects of quince (dwarfing) and Pyrus calleryana (vigorous) on pear scions, reported that the least vigorous rootstocks produced the highest number of floral buds. In this regard, Fazio et al.26 reported that the Quantitative Trait Locus or Loci (QTL) for early bearing co-located with the QTL for scion vigor suppression (dwarfing). Therefore, the relationship between low vigor and precocity would be expected due to linkage disequilibrium.
In the case of overexpression of PpCBF1 in the rootstock resulting in reduced growth and delayed flowering, the cause must be due to some commonality between the two processes rather than from some random effect on genes located in the region of the QTLs for either dwarfing or early bearing. This is supported by the fact that several independent PpCBF1 overexpressing lines, representing separate random insertion events, all resulted to a similar degree in the same apple phenotype.10 As previously noted, Wisniewski et al.12 demonstrated that overexpression of PpCBF1 in M.26 apple resulted in elevated levels of RGL (DELLA) genes during the time period when growth rate is very high and that these two factors (overexpression of PpCB1 and RGL genes) were associated with reduced growth. It is also known that CBF overexpression can reduce the level of GA precursors43 and possibly increase the catabolism of bio-active GAs through GA2ox.23 In addition to their known growth-repressive activities, DELLA (RGL) proteins have also been reported to affect several portions of floral induction pathways. A schematic diagram of how CBF may influence flowering and other developmental parameters is presented in Figure 8. This schematic expands on the model presented by Wisniewski et al.12 illustrating how CBF overexpression could modulate freezing tolerance, dormancy, growth and indirectly floral induction. DELLA levels antagonize SPL (Squamosa promoter binding like) proteins, which positively regulate one of the pathways of floral induction.22 SPLs are a component of the miR156 (micro RNA 156) mediated-age pathway in Arabidopsis, with SPL levels gradually increasing during the transition from the juvenile to adult states.45 Specific phytochrome interacting factors (PIFs) integrate light and GA signals to regulate another floral induction pathway. Downstream of the PIF/GA interaction are GNC/GNL proteins (GNC: GATA, nitrate-inducible, carbon-metabolism involved; GNL: GNC-like), which act as repressors of the flowering time agent, SOC (suppressor of overexpression of constans 1), in Arabidopsis.46 DELLAs act via repression of PIF, thus maintaining repression of floral (and other developmental pathways) by GNC/GNL.45,46 SOC, is a known component of a floral induction pathway including CO (constans), FT (flowering time) and the floral meristem identity protein, LFY (leafy)47. In addition, decreased levels of bio-active GAs may contribute toward reduced levels of FT.47,48 Apple homologs of SPL and GNC/GNL genes were identified by BLAST searches of the apple genome in the Genome Database for Rosaceae (GDR, http://www.rosaceae.org/) (data not shown).49,50
Schematic diagram of the potential influence of CBF gene expression on the regulation of freezing tolerance, dormancy, growth and juvenility (time to flowering). In the model, CBF gene expression regulates RGL (DELLA) gene expression which then impacts expression of growth and flowering-related pathways. CBF gene overexpression directly affects freezing tolerance and dormancy. Solid lines with arrowheads indicate positive regulation; solid lines ending as a ‘T’ indicate negative regulation; dotted line with arrowhead indicates GA inactivation pathway; ‘+’ indicates interaction of GA with PIF. AP-2, APETALA-2; COR, cold regulated; EBB, early budbreak; GNC/GNL, GNC: GATA, nitrate-inducible, carbon-metabolism involved; GNL, GNC-like; GAMYB, GA-regulated MYB transcription factor; miR156 and miR172, micro RNAs; TFs, transcription factors. Figure based on models presented in various reports.12,22,23,41,45–48,51–55
A potential argument against the model presented in Figure 8 is that in some studies GA has been reported to inhibit floral induction in apple, especially when exogenous GA is applied.51,52 However, in a review of the available literature, Bangerth52 indicated that ambiguities exist when regarding the effects of endogenous GA on floral induction in fruit trees.
In conclusion, the current study examined the ability of transgenic T166 rootstocks to impact various physiological and developmental properties of ‘Royal Gala’ which was used as a scion and grafted on the T166 rootstock. Dormancy, freezing tolerance, growth and flowering data were recorded for RG/T166 and RG/M.26 trees and compared with each other and with data recorded for own-rooted and self-grafted M.26 and T166 trees. Dormancy (as measured by budset, leaf senescence and spring budbreak) and freezing tolerance were similar in RG/T166 and RG/M.26 trees. No evidence of graft transmission of the PpCBF1 transgene was observed. In contrast, both growth and precocity (time to flowering) were inhibited and delayed, respectively, in RG/T166 trees compared with RG/M.26 trees. Elevated levels of DELLA (RGL) mRNA in scions grafted to transgenic rootstocks may partially account for the observed differences in growth and flowering.
References
Thomashow MF . Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol 2010; 154: 571–577.
Medina J, Catalá R, Salinas J . The CBFs: three arabidopsis transcription factors to cold acclimate. Plant Sci 2011; 180: 3–11.
Qin F, Shinozaki K, Yamaguchi-Shinozaki K . Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 2011; 52: 1569–1582.
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K . AP2/ERF transcription factors in plant abiotic stress responses. Biochim Biophys Acta 2012; 1819: 86–96.
Welling A, Palva ET . Involvement of CBF transcription factors in winter hardiness in birch. Plant Physiol 2008; 147: 1199–1211.
Benedict C, Skinner JS, Meng R, Chang Y, Bhalerao R, Huner NP et al. The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ 2006; 29: 1259–1272.
Xiao H, Siddiqua M, Braybrook S, Nassuth A . Three grape CBF/DREB1 genes are regulated by low temperature, drought and abscisic acid. Plant Cell Environ 2006; 29: 1410–1421.
Navarro M, Ayax C, Martinez Y, Laur J, El Kayal W, Marque C et al. Two EguCBF1 genes overexpressed in Eucalyptus display a different impact on stress tolerance and plant development. Plant Biotechnol J 2011; 9: 50–63.
Wisniewski M, Nassuth A, Teulieres C, Marque C, Rowland J, Cao PB et al. Genomics of cold hardiness in woody plants. Crit Rev Plant Sci 2014; 33: 92–124.
Wisniewski M, Norelli J, Bassett C, Artlip T, Macarisin D . Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus×domestica) results in short-day induced dormancy and increased cold hardiness. Planta 2011; 233: 971–983.
Artlip TS, Wisniewski ME, Bassett CL, Norelli JL . CBF gene expression in peach leaf and bark tissues is gated by a circadian clock. Tree Phys 2013; 33: 866–877.
Wisniewski M, Norelli J, Artlip T . Overexpression of a peach CBF in apple: a model for understanding the integration of growth, dormancy, and cold hardiness in woody plants. Front Plant Sci 2015; 6: 85.
Hsieh T-H, Lee J-T, Yang P-T, Chiu LH, Charng YY, Wang YC et al. Heterologous expression of the Arabidopsis C-repeat/dehydration response element 1 confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol 2002; 129: 1086–1094.
Pino M-T, Skinner JS, Jeknić Z, Hayes PM, Soeldner AH, Thomashow MF et al. Ectopic AtCBF1 over-expression enhances freezing tolerance and induces cold acclimation associated physiological modifications in potato. Plant Cell Environ 2008; 31: 393–406.
Tillett RL, Wheatley MD, Tattersall EAR, Schlauch KA, Cramer GR, Cushman JC . The Vitis vinifera C-repeat binding protein4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Biotechnol J 2011; 10: 105–124.
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998; 10: 1391–1406.
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K . Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress- inducible transcription factor. Nat Biotechnol 1999; 17: 287–291.
Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF . Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 2000; 124: 1854–1865.
Gilmour SJ, Fowler SG, Thomashow MF . Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol Biol 2004; 54: 767–781.
Artlip TS, Wisniewski ME, Norelli JL . Field evaluation of apple overexpressing a peach CBF Gene confirms its effect on cold hardiness, dormancy, and growth. Environ Exp Bot 2014; 106: 79–86.
Heide OM, Prestrud AK . Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol 2005; 25: 109–114.
Xu H, Liu Q, Yao T, Fu X . Shedding light on integrative GA signaling. Curr Opin Plant Biol 2014; 21: 89–95.
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P . The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008; 20: 2117–2129.
Yordanov YS, Ma C, Strauss SH, Busov VB . EARLY BUD-BREAK 1 (EBB1) is a regulator of release from seasonal dormancy in poplar trees. Proc Natl Acad Sci USA 2014; 111: 10001–10006.
Van Hooijdonk B, Woolley D, Warrington I . Rootstocks modify scion architecture, endogenous hormones, and root growth of newly grafted ‘Royal Gala’ apple trees. J Am Soc Hort Sci 2011; 136: 93–102.
Fazio G, Wan Y, Kviklys D, Romero L, Adams R, Strickland D et al. Dw2, a new dwarfing locus in apple rootstocks and its relationship to induction of early bearing in apple scions. J Am Soc Hort Sci 2014; 139: 87–98.
Haroldsen VM, Szczerba MW, Aktas H, Lopez-Baltazar J, Odias MJ, Chi-Ham CL et al. Mobility of transgenic nucleic acids and proteins within grafted rootstocks for agricultural improvement. Front Plant Sci 2012; 3: 39.
Zhao D, Song G-Q . Rootstock-to-scion transfer of transgene-derived small interfering RNAs and their effect on virus resistance in nontransgenic sweet cherry. Plant Biotech J 2014; 12: 1319–1328.
Aguero CB, Uratsu SL, Greve C, Powell AL, Labavitch JM, Meredith CP et al. Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol 2005; 6: 43–51.
Dutt M, Li ZT, Kelley KT, Dhekney SA, Van Aman M, Tattersall J et al. Transgenic rootstock protein transmission in grapevines. Acta Hort 2007; 738: 749–753.
Norelli JL, Aldwinckle HS, Beer SV . Leaf wounding increases efficiency of Agrobacterium-mediated transformation of apple. Phytopathology 1988; 78: 1292–1297.
Ko K, Norelli JL, Reynoird J-P, Aldwinckle HS, Brown SK . T4 lysozyme and attacin genes enhance resistance of transgenic ‘Galaxy’ apple against Erwinia amylorvora . J Am Soc Hort Sci 2002; 127: 515–519.
Bolar JP, Hanke V, Norelli JL, Aldwinckle HS . An efficient method for rooting and acclimation of micropropagated apple cultivars. HortScience 1998; 33: 1251–1252.
Zhang MIN, Willison JHM . An improved conductivity method for the measurement of frost hardiness. Can J Bot 1987; 65: 710–715.
Bowen J, Ireland HS, Crowhurst R et al. Selection of low-variance expressed Malus×domestica (apple) genes for use as quantitative PCR reference genes (housekeepers). Tree Genet Genomes 2014; 10: 751–759.
Anderson CL, Jensen JL, Ømtoft TF . Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 2004; 64: 5245–5250.
Chapman PJ, Catlin GA . Growth stages in fruit trees—from dormant to fruit set. New York Food Life Sci Bull 1976 No. 58: 1–11.
Howe GT, Gardner G, Hackett WP, Furnier GR, Cordonnier-Pratt MM, Gardner G . Phytochrome control of short-day-induced bud set in black cottonwood. Phys Plant 1996; 97: 95–103.
Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF . Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998; 280: 104–106.
Watson AE, Seleznyova AN, Dayatilake GA, Tustin DS . Rootstocks affect pear (Pyrus communis) tree growth through extent of node neoformation and flowering with key differences to apple. Funct Plant Biol 2012; 39: 493–502.
Achard P, Genschik P . Releasing the brakes of plant growth: how GAs shutdown DELLA proteins. J Exp Bot 2009; 60: 1085–1092.
Suo H, Ma Q, Ye K, Yang C, Tang Y, Hao J et al. Overexpression of AtDREB1A causes a severe dwarf phenotype by decreasing endogenous gibberellin levels in soybean [Glycine max (L.) Merr.]. PLoS One 2012; 7: e45568.
Niu S, Gao Q, Li Z, Chen X, Li W . The role of gibberellin in the CBF1-mediated stress-response pathway. Plant Mol Biol Rep 2014; 32: 852–863.
Xu H, Zhang W, Li M, Harada T, Han Z, Li T . Gibberellic acid insensitive mRNA transport in both directions between stock and scion in Malus . Tree Gen Genet 2010; 6: 1013–1019.
Wang J-W . Regulation of flowering time by the miR156-mediated age pathway. J Exp Bot 2014; 65: 4723–4730.
Richter R, Behringer C, Müller IK, Schwechheimer C . The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME INTERACTINGFACTORS. Gene Dev 2010; 24: 2093–2104.
Mutasa-Göttgens E, Hedden P . Giberellin as a factor in floral regulatory networks. J Exp Bot 2009; 60: 1979–1989.
Pajoro A, Biewers S, Dougali E, Leal Valentim F, Mendes MA, Porri A et al. The (r)evolution of gene regulatory networks controlling Arabidopsis plant reproduction: a two-decade history. J Exp Bot 2014; 65: 4731–4745.
Thompson JD, Higgins DG, Gibson TJ . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22: 4673–4680.
Jung S, Ficklin S, Lee T, Cheng CH, Blenda A, Zheng P et al. The Genome Database for Rosaceae (GDR): year 10 update. Nucleic Acids Res 2014; 42: D1237–D1244.
Kurokura T, Mimida N, Battey NH, Hytönen T . The regulation of seasonal flowering in the Rosaceae. J Exp Bot 2013; 64: 4131–4141.
Bangerth KF . The induction in mature, perennial angiosperm fruit trees: similarities and discrepancies with annual/ biennial plants and the involvement of plant hormones. Sci Hort 2009; 122: 153–163.
Solofoharivelo MC, van der Walt AP, Stephan D, Burger JT, Murray SL . MicroRNAs in fruit trees: discovery, diversity and future research directions. Plant Biol 2014; 16: 856–865.
Foster TM, Watson AE, van Hooijdonk BM, Schaffer RJ . Key flowering genes including FT-like genes are upregulated in the vasculature of apple dwarfing rootstocks. Tree Genet Genomes 2014; 10: 189–202.
Zhao Q, Su C, Liu D-D, Hao Y-J, You C-X . Ectopic expression of the apple Md-miR172e gene alters flowering time and floral organ identity in Arabidopsis . Plant Cell Tissue Org Culture 2015; 123: 535–546.
Acknowledgements
We thank Phil Welser for his technical assistance in tissue culture, grafting, field maintenance, and gene expression data and Erik Burchard for his technical assistance with the gene expression data. Mark DeMuth and Doug Raines are thanked for their assistance with the bud grafting. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplemental Information for this article can be found on the Horticulture Research website (http://www.nature.com/hortres).
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Artlip, T., Wisniewski, M., Arora, R. et al. An apple rootstock overexpressing a peach CBF gene alters growth and flowering in the scion but does not impact cold hardiness or dormancy. Hortic Res 3, 16006 (2016). https://doi.org/10.1038/hortres.2016.6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hortres.2016.6
This article is cited by
-
Development of genetically modified sweet cherry rootstock ‘Gisela 6’ with overexpression of PcMPK3-HA gene by Agrobacterium-mediated genetic transformation
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
Differential gene expression in non-transgenic and transgenic “M.26” apple overexpressing a peach CBF gene during the transition from eco-dormancy to bud break
Horticulture Research (2019)
-
A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants
Horticulture Research (2018)
-
Transcriptomic changes reveal gene networks responding to the overexpression of a blueberry DWARF AND DELAYED FLOWERING 1 gene in transgenic blueberry plants
BMC Plant Biology (2017)
-
Mining and expression analysis of candidate genes involved in regulating the chilling requirement fulfillment of Paeonia lactiflora ‘Hang Baishao’
BMC Plant Biology (2017)