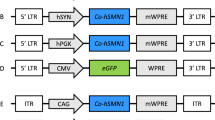
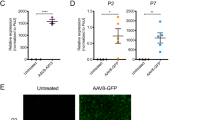
Abstract
The reduced level of survival motor neuron (SMN) protein, caused by homozygous deletions in the SMN gene, led to a common neurodegenerative disorder known as spinal muscular atrophy (SMA). In spite of extensive efforts to find a cure for SMA, there is currently no effective treatment available for this devastating disease. In this study, restoration of SMN expression through ‘gene-targeting’ method in SMA fibroblast cells was attempted. We designed a 2697-bp gene-targeting cassette; it consisted of an SMN1 open reading frame expressing 38 kD SMN protein and the upstream and downstream regions of exon 1 of SMN1 gene at the ends as the homology arms. SMA fibroblast cells were transfected by gene-targeting cassette using Lipofectamine LTX-PLUS reagent. Occurrence of homologous recombination in selected cells was investigated by PCR analysis. Increased expression of SMN protein was shown by real-time PCR and western blotting analysis. The immunofluorescence analysis results demonstrated that the number of SMN nuclear structures, Gems, was the same as or greater than the number of Gems found in normal fibroblasts. The results of this study indicate that gene-targeting methods do, in fact, present as an alternative for restoration of SMN expression in SMA patients-derived cells in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pearn J . Classification of spinal muscular atrophies. Lancet 1980; 1: 919–922.
Munsat TL, Davies KE . International SMA Consortium meeting. Neuromuscul Disord 1992; 2: 423–428.
Zerres K, Rudnik-Schöneborn S . Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol 1995; 52: 518–523.
Bassell W, Spinal GJ . Muscular atrophy and a model for survival of motor neuron protein function in axonal ribonucleoprotein complexes. Results Probl Cell Differ 2009; 48: 289–326.
Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AHM et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Molec Genet 1999; 8: 1177–1183.
Boda B, Mas C, Giudicelli C, Nepote V, Guimiot F, Levacher B et al. Survival motor neuron SMN1 and SMN2 gene promoters: identical sequences and differential expression in neurons and non-neuronal cells. Eur J Hum Genet 2004; 12: 729–737.
Lorson CL, Rindt H, Shababi M . Spinal muscular atrophy: mechanisms and therapeutic strategies. Hum Mol Gene 2010; 19: R111–R118.
Mulcahy PJ, Iremonger K, Karyka E, Herranz-Martín S, Shum KT, Tam JK et al. Gene therapy: a promising approach to treating spinal muscular atrophy. Hum Gene Ther 2014; 25: 575–586.
DiDonato CJ, Parks RJ, Kothary R . Development of a gene therapy strategy for the restoration of survival motor neuron protein expression: implications for spinal muscular atrophy therapy. Hum Gene Ther 2003; 14: 179–188.
Azzouz M, Le T, Ralph GS, Walmsley L, Monani UR, Lee DC et al. Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy. J Clin Invest 2004; 114: 1726–1731.
Baughan T, Shababi M, Coady TH, Dickson AM, Tullis GE, Lorson CL . Stimulating full-length SMN2 expression by delivering bifunctional RNAs via a viral vector. Mol Ther 2006; 14: 54–62.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK . Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 2009; 27: 59–65.
Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O’Riordan CR et al. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Invest 2010; 120: 1253–1264.
Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 2010; 28: 271–274.
Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet 2011; 20: 681–693.
Glascock JJ, Osman EY, Wetz MJ, Krogman MM, Shababi M, Lorson CL . Decreasing disease severity in symptomatic, Smn−/−;SMN2+/+, spinal muscular atrophy mice following scAAV9-SMN delivery. Hum Gene Ther 2012; 23: 330–335.
Benkhelifa-Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R et al. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol Ther 2013; 21: 282–290.
Passini MA, Bu J, Richards AM, Treleaven CM, Sullivan JA, O'Riordan CR et al. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum Gene Ther 2014; 25: 619–630.
Vannucci L, Lai M, Chiuppesi F, Ceccherini-Nelli L, Pistello M . Viral vectors: a look back and ahead on gene transfer technology. New Microbiol 2013; 36: 1–22.
Primrose SB, Twyman RM, Old RW . Principles of Gene Manipulation. Blackwell Science Ltd: London, UK, 2001.
Kan Y, Ruis B, Lin S, Hendrickson EA . The mechanism of gene targeting in human somatic cells. PLoS Genetics 2014; 10: 1–14.
Thomas LS . Gene targeting vector design for embryonic stem cell modifications. In: Pease S, Saunders TL (eds). Advanced Protocols for Animal Transgenesis. Springer: Heidelberg, Germany; New York, USA, 2011, pp 57–79.
Sorrell AD, Kolb FA . Targeted modification of mammalian genomes. Biotechnol Adv 2005; 23: 431–469.
Muller U . Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev 1999; 82: 3–21.
Lanzov VA . Gene targeting for gene therapy: prospects. Mole Genet Metab 1999; 68: 276–282.
Narsinh KH, Wu JC . Gene correction in human embryonic and induced pluripotent stem cells: promises and challenges ahead. Mol Ther 2010; 18: 1061–1063.
Khanahmad H, Noori Daloii MR, Shokrgozar MA, Azadmanesh K, Niavarani AR, Karimi M et al. A novel single step double positive double negative selection strategy for b-globin gene replacement. Biochem Biophys Res Commun 2006; 345: 14–20.
Lam AP, Dean DA . Progress and prospects: nuclear import of nonviral vectors. Gene Therapy 2010; 17: 439–447.
Vaughan EE, DeGiulio JV, Dean DA . Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import. Curr Gene Ther 2006; 6: 671–681.
Dormiani K, Sadeghi HMM, Sadeghi-Aliabadi H, Ghaedi K, Forouzanfar M, Baharvand H et al. Long-term and efficient expression of human b-globin gene in a hematopoietic cell line using a new sitespecific integrating non-viral system. Gene Ther 2015, e-pub ahead of print 1 April 2015; doi:10.1038/gt.2015.30.
Dean DA, Strong DD, Zimmer WE . Nuclear entry of nonviral vectors. Gene Therapy 2005; 12: 881–890.
Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev 2010; 24: 1634–1644.
DiMatteo D, Callahan S, Kmiec EB . Genetic conversion of an SMN2 gene to SMN1: a novel approach to the treatment of spinal muscular atrophy. Exp Cell Res 2008; 314: 878–886.
Corti S, Nizzardo M, Simone C, Falcone M, Nardini M, Ronchi D et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Transl Med 2012; 4: 165ra162.
Sangiuolo F, Filareto A, Spitalieri P, Scaldaferri ML, Mango R, Bruscia E et al. In vitro restoration of functional SMN protein in human trophoblast cells affected by spinal muscular atrophy by small fragment homologous replacement. Hum Gene Ther 2005; 16: 869–880.
Jablonka S, Bandılla M, Wıese S, Buhler D, Wırth B, Sendtner M et al. Co-regulation of survival of motor neuron (SMN) protein and its interactor SIP1 during development and in spinal muscular atrophy. Hum Mol Genet 2001; 10: 497–505.
Jablonka S, Holtmann B, Meıster G, Bandılla M, Rossoll W, Fıscher U et al. Gene targeting of Gemin2 in mice reveals a correlation between defects in the biogenesis of U snRNPs and motoneuron cell death. Proc Natl Acad Sci USA 2002; 99: 10126–10131.
Arnold AS, Gueye M, Rondé P, Warter JM, Poindron P, Gies JP . Construction of a plasmid containing human SMN, the SMA determining gene, coupled to EGFP. Plasmid 2002; 47: 79–87.
Volodin AA, Voloshin ON, Camerini-Otero RD . Homologous recombination and RecA protein: towards a new generation of tools for genome manipulations. Trends Biotechnol 2005; 23: 97–102.
Mani M, Kandavelou K, Dy JF, Duria S, Chandrasegaran S . Design, engineering, and characterization of zinc finger nucleases. Biochem Biophys Res Commun 2005; 335: 447–457.
Connelly JP, Barker JC, Pruett-Miller S, Porteus MH . Gene correction by homologous recombination with zinc finger nucleases in primary cells from a mouse model of a generic recessive genetic disease. Mol Ther 2010; 18: 1103–1110.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F . Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013; 8: 2281–2308.
Hanahan D . Studies on transformation on E. coli with plasmids. J Mol Biol 1983; 166: 557–580.
Acknowledgements
We thank the Ege University Scientific Research Projects (APAK) for financial support (Grant Number 2012/TIP/033). We also express our sincere appreciation to Professor Dr Bahram Kazemi (Director of Medical Biotechnology Department, School of Medicine, Shahid Beheshti University of Medical Sciences) for assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rashnonejad, A., Gündüz, C., Süslüer, S. et al. In vitro gene manipulation of spinal muscular atrophy fibroblast cell line using gene-targeting fragment for restoration of SMN protein expression. Gene Ther 23, 10–17 (2016). https://doi.org/10.1038/gt.2015.92
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.92
This article is cited by
-
Restoration of SMN expression in mesenchymal stem cells derived from gene-targeted patient-specific iPSCs
Journal of Molecular Histology (2018)