Abstract
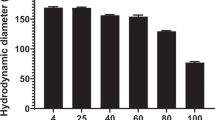
A novel class of PEGylated polyacridine peptides was developed that mediate potent stimulated gene transfer in the liver of mice. Polyacridine peptides, (Acr-X)n-Cys-polyethylene glycol (PEG), possessing 2–6 repeats of Lys-acridine (Acr) spaced by either Lys, Arg, Leu or Glu, were Cys derivatized with PEG (PEG5000 kDa) and evaluated as in vivo gene transfer agents. An optimal peptide of (Acr-Lys)6-Cys-PEG was able to bind to plasmid DNA (pGL3) with high affinity by polyintercalation, stabilize DNA from metabolism by DNAse and extend the pharmacokinetic half-life of DNA in the circulation for up to 2 h. A tail vein dose of PEGylated polyacridine peptide pGL3 polyplexes (1 μg in 50 μl), followed by a stimulatory hydrodynamic dose of normal saline at times ranging from 5 to 60 min post-DNA administration, led to a high level of luciferase expression in the liver, equivalent to levels mediated by direct hydrodynamic dosing of 1 μg of pGL3. The results establish the unique properties of PEGylated polyacridine peptides as a new and promising class of gene delivery peptides that facilitate reversible binding to plasmid DNA, protecting it from DNase in vivo resulting in an extended circulatory half-life, and release of transfection-competent DNA into the liver to mediate a high-level of gene expression upon hydrodynamic boost.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Burke RS, Pun SH . Extracellular barriers to in vivo PEI and PEGylated PEI polyplex-mediated gene delivery to the liver. Bioconjug Chem 2008; 19: 693–704.
Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M . Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med 2004; 6: 1102–1111.
Rudolph C, Schillinger U, Plank C, Gessner A, Nicklaus P, Muller R et al. Nonviral gene delivery to the lung with copolymer-protected and transferrin-modified polyethylenimine. Biochim Biophys Acta 2002; 1573: 75–83.
Guillaume C, Delepine P, Droal C, Montier T, Tymen G, Claude F . Aerosolization of cationic lipid–DNA complexes: lipoplex characterization and optimization of aerosol delivery conditions. Biochem Biophys Res Commun 2001; 286: 464–471.
Mahato RI, Anwer K, Tagliaferri F, Meaney C, Leonard P, Wadhwa MS et al. Biodistribution and gene expression of lipid/plasmid complexes after systemic administration. Hum Gene Ther 1998; 9: 2083–2099.
Li S, Rizzo MA, Bhattacharya S, Huang L . Characterization of cationic lipid–protamine–DNA (LPD) complexes for intravenous gene delivery. Gene Therapy 1998; 5: 930–937.
Liu F, Qi H, Huang L, Liu D . Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration. Gene Therapy 1997; 4: 517–523.
Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J et al. Improved cationic lipid formulations for in vivo gene therapy. Ann N Y Acad Sci 1995; 772: 126–139.
Al-Dosari MS, Knapp JE, Liu D, Leaf Huang M-ChaEw . Hydrodynamic delivery. In: Adv Genet, vol. 54. Academic Press, 2005, pp 65–82.
Liu F, Song Y, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999; 6: 1258–1266.
Molnar MJ, Gilbert R, Lu Y, Liu AB, Guo A, Larochelle N et al. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol Ther 2004; 10: 447–455.
Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL . Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Therapy 2003; 10: 1608–1615.
Fattori E, La Monica N, Ciliberto G, Toniatti C . Electro-gene-transfer: a new approach for muscle gene delivery. Somat Cell Mol Genet 2002; 27: 75–83.
Li S, MacLaughlin FC, Fewell JG, Gondo M, Wang J, Nicol F et al. Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid. Gene Therapy 2001; 8: 494–497.
Liu F, L H . A syringe electrode device for simultaneous injection of DNA and electrotransfer. Mol Ther 2002; 5: 323–328.
Heyes J, Palmer L, Chan K, Giesbrecht C, Jeffs L, MacLachlan I . Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Mol Ther 2007; 15: 713–720.
Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E . PEGylated DNA/transferrin–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Therapy 1999; 6: 595–605.
Blessing T, Kursa M, Holzhauser R, Kircheis R, Wagner E . Different strategies for formation of PEGylated EGF-conjugated PEI/DNA complexes for targeted gene delivery. Bioconj Chem 2001; 12: 529–537.
Mannisto M, Vanderkerken S, Toncheva V, Elomaa M, Ruponen M, Schacht E et al. Structure–activity relationships of poly(L-lysines): effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J Control Rel 2002; 83: 169–182.
Kwok KY Park Y, Yongsheng Y, McKenzie DL, Rice KG . In vivo gene transfer using sulfhydryl crosslinked PEG-peptide/glycopeptide DNA co-condensates. J Pharm Sci 2003; 92: 1174–1185.
Yang Y, Park Y, Man S, Liu Y, Rice KG . Cross-linked low molecular weight glycopeptide-mediated gene delivery: relationship between DNA metabolic stability and the level of transient gene expression in vivo. J Pharm Sci 2001; 90: 2010–2022.
Collard WT, Yang Y, Kwok KY, Park Y, Rice KG . Biodistribution, metabolism, and in vivo gene expression of low molecular weight glycopeptide polyethylene glycol peptide DNA co-condensates. J Pharm Sci 2000; 89: 499–512.
Nishikawa M, Yamauchi M, Morimoto K, Ishida E, Takakura Y, Hashida M . Heptocyte-targeted in vivo gene expression by intravenous injection of plasmid DNA complexed with synthetic multi-functional gene delivery system. Gene Therapy 2000; 7: 548–555.
Schuster MJ, Wu GY, Walton CM, Wu CH . Multicomponent DNA carrier with a vesicular stomatitis virus G-peptide greatly enhances liver-targeted gene expression in mice. Bioconj Chem 1999; 10: 1075–1083.
Zhang G, Budker V, Wolff JA . High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 1999; 10: 1735–1737.
Dash PR, Read ML, Barrett LB, Wolfert MA, Seymour LW . Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Therapy 1999; 6: 643–650.
Ueyama H, Waki M, Takagi M, Takenaka S . Novel synthesis of a tetra-acridinyl peptide as a new DNA polyintercalator. Nucleic Acids Symp Ser 2000; 44: 133–134.
Haensler J, Szoka JFC . Synthesis and characterization of a trigalactosylated bisacridine compound to target DNA to hepatocytes. Bioconjug Chem 1993; 4: 85–93.
Boulanger C, Di Giorgio C, Vierling P . Synthesis of acridine-nuclear localization signal (NLS) conjugates and evaluation of their impact on lipoplex and polyplex-based transfection. Eur J Med Chem 2005; 40: 1295–1306.
Shiraishi T, Hamzavi R, Nielsen PE . Targeted delivery of plasmid DNA into the nucleus of cells via nuclear localization signal peptide conjugated to DNA intercalating bis- and trisacridines. Bioconj Chem 2005; 16: 1112–1116.
Vinogradov SV, Zhang H, Mitin A, Warren G . Intercalating conjugates of Peg with nuclear localization signal (Nls) peptide. Polymer Prep 2008; 49: 434–435.
Zhang H, Mitin A, Vinogradov SV . Efficient transfection of blood–brain barrier endothelial cells by lipoplexes and polyplexes in the presence of nuclear targeting NLS-–EG–acridine conjugates. Bioconjug Chem 2009; 20: 120–128.
Zhang H, Vinogradov SV . Short biodegradable polyamines for gene delivery and transfection of brain capillary endothelial cells. J Control Rel 2010; 143: 359–366.
Baumhover NJ, Anderson K, Fernandez CA, Rice KG . Synthesis and in vitro testing of new potent polyacridine–melittin gene delivery peptides. Bioconj Chem 2010; 21: 74–86.
Anderson K, Fernandez CA, Rice KG . N-Glycan targeted gene delivery to the dendritic cell SIGN receptor. Bioconj Chem 2010, e-pub ahead of print 22 May 2010, DOI: 10.1021/bc1000824.
Fernandez CA, Baumhover NJ, Anderson K, Rice KG . Discovery of metabolically stablized electronegative polyacridine–PEG peptide DNA open polyplexes. Bioconj Chem 2010; 21: 723–730.
Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA . A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther 1996; 7: 1947–1954.
Blessing T, Remy JS, Behr JP . Monomolecular collapse of plasmid DNA into stable virus-like particles. Proc Natl Acad Sci USA 1998; 95: 1427–1431.
Kwok KY, McKenzie DL, Evers DL, Rice KG . Formulation of highly soluble poly(ethylene glycol)-peptide DNA condensates. J Pharm Sci 1999; 88: 996–1003.
Chen CP, Kim JS, Liu D, Rettig GR, McAnuff MA, Martin ME et al. Synthetic PEGylated glycoproteins and their utility in gene delivery. Bioconj Chem 2007; 18: 371–378.
Kunath K, von Harpe A, Petersen H, Fischer D, Voigt K, Kissel T et al. The structure of PEG-modified poly(ethylene imines) influences biodistribution and pharmacokinetics of their complexes with NF-κB decoy in mice. Pharm Res 2002; 19: 810–817.
Li S-D, Chen Y-C, Hackett MJ, Huang L . Tumor-targeted delivery of siRNA by Self-assembled Nanoparticles. Mol Ther 2007; 16: 163–169.
Morille M, Montier T, Legras P, Carmoy N, Brodin P, Pitard B et al. Long-circulating DNA lipid nanocapsules as new vector for passive tumor targeting. Biomaterials 2010; 31: 321–329.
Oupicky D, Ogris M, Howard KA, Dash PR, Ulbrich L, Seymour LW . Importance of lateral an steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol Ther 2002; 5: 463–472.
Ko YT, K A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP . Self-assembling micelle-like nanoparticles based on phospholipid–polyethyleneimine conjugates for systemic gene delivery. J Control Rel 2009; 133: 132–138.
Zhou Q-H, Wu C, Manickam D, Oupický D . Evaluation of pharmacokinetics of bioreducible gene delivery vectors by real-time PCR. Pharm Res 2009; 26: 1581–1589.
Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG . Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconj Chem 1997; 8: 81–88.
Adami RC, Rice KG . Metabolic stability of glutaraldehyde cross-linked peptide DNA condensates. J Pharm Sci 1999; 88: 739–746.
Terebesi J, Kwok KY, Rice KG . Iodinated plasmid DNA as a tool for studying gene delivery. Anal Biochem 1998; 263: 120–123.
Rettig G, McAnuff M, Kim J, Liu D, Rice KG . Quantitative bioluminescence imaging of transgene expression in vivo. Anal Biochem 2006; 335: 90–94.
Acknowledgements
The authors gratefully acknowledge support from NIH Grant DK066212.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fernandez, C., Baumhover, N., Duskey, J. et al. Metabolically stabilized long-circulating PEGylated polyacridine peptide polyplexes mediate hydrodynamically stimulated gene expression in liver. Gene Ther 18, 23–37 (2011). https://doi.org/10.1038/gt.2010.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.117
Keywords
This article is cited by
-
Targeted nano-delivery of chemotherapy via intranasal route suppresses in vivo glioblastoma growth and prolongs survival in the intracranial mouse model
Drug Delivery and Translational Research (2023)
-
Heat-shrinking DNA nanoparticles for in vivo gene delivery
Gene Therapy (2020)
-
Efficient expression of stabilized mRNA PEG-peptide polyplexes in liver
Gene Therapy (2015)
-
Research on redox-responsive mesoporous silica nanoparticles functionalized with PEG via a disulfide bond linker as drug carrier materials
Colloid and Polymer Science (2015)
-
The uptake mechanism of PEGylated DNA polyplexes by the liver influences gene expression
Gene Therapy (2014)