Abstract
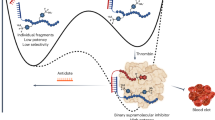
The particle size of a PEG-peptide DNA nanoparticle is a key determinant of biodistribution following i.v. dosing. DNA nanoparticles of <100 nm in diameter are sufficiently small to cross through fenestrated endothelial cells to target hepatocytes in the liver. In addition, DNA nanoparticles must be close to charge-neutral to avoid recognition and binding to scavenger receptors found on Kupffer cells and endothelial cells in the liver. In the present study, we demonstrate an approach to heat shrink DNA nanoparticles to reduce their size to <100 nm to target hepatocytes. An optimized protocol heated plasmid DNA at 100 °C for 10 min resulting in partial denaturation. The immediate addition of a polyacridine PEG-peptide followed by cooling to room temperature resulted in heat-shrunken DNA nanoparticles that were ~70 nm in diameter compared with 170 nm when heating was omitted. Heat shrinking resulted in the conversion of supercoiled DNA into open circular to remove strain during compaction. Heat-shrunken DNA nanoparticles were stable to freeze-drying and reconstitution in saline. Hydrodynamic dosing established that 70 nm heat-shrunken DNA nanoparticles efficiently expressed luciferase in mouse liver. Biodistribution studies revealed that 70 nm DNA nanoparticles are rapidly and transiently taken up by liver whereas 170 nm DNA nanoparticles avoid liver uptake due to their larger size. The results provide a new approach to decrease the size of polyacridine PEG-peptide DNA nanoparticles to allow penetration of the fenestrated endothelium of the liver for the purpose of transfecting hepatocytes in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–301.
Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug Chem. 1997;8:81–8.
Wagner E, Zenke M, Cotten M, Beug H, Birnstiel ML. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci USA. 1990;87:3410–4.
Kabanov AV, Kabanov VA. DNA complexes with polycations for the delivery of genetic material into cells. Bioconjug Chem. 1995;6:7–20.
Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–33.
Bettinger T, Remy JS, Erbacher P. Size reduction of galactosylated PEI/DNA complexes improves lectin-mediated gene transfer into hepatocytes. Bioconjug Chem. 1999;10:558–61.
Park SY, Harriers D, Gelbart WM. Topological defects and optimum size of DNA condensates. Biophys. J. 1998;75:714–20.
Erbacher P, Roche AC, Monsigny M, Midoux P. Glycosylated polylysine/DNA complexes: gene transfer efficiency in relation with the size and the sugar substitution level of glycosylated polylysines and with the plasmid size. Bioconjug Chem. 1995;6:401–10.
Fink TL, Klepcyk PJ, Oette SM, Gedeon CR, Hyatt SL, Kowalczyk TH, et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13:1048–51.
Kaminskas LM, Boyd BJ, Karellas P, Krippner GY, Lessene R, Kelly B, et al. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly l-lysine dendrimers. Mol Pharm. 2008;5:449–63.
Fernandez CA, Baumhover NJ, Duskey JT, Khargharia S, Kizzire K, Ericson MD, et al. Metabolically stabilized long-circulating PEGylated polyacridine peptide polyplexes mediate hydrodynamically stimulated gene expression in liver. Gene Ther. 2011;18:23–37.
Kwok KY, McKenzie DL, Evers DL, Rice KG. Formulation of highly soluble poly(ethylene glycol)-peptide DNA condensates. J Pharm Sci. 1999;88:996–1003.
Pezzoli D, Giupponi E, Mantovani D, Candiani G. Size matters for in vitro gene delivery: investigating the relationships among complexation protocol, transfection medium, size and sedimentation. Sci Rep. 2017;7:44134.
Hickey JW, Santos JL, Williford JM, Mao HQ. Control of polymeric nanoparticle size to improve therapeutic delivery. J Control Release. 2015;219:536–47.
Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, et al. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278:32578–86.
Prabha S, Zhou W, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm. 2002;244:105–15.
Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–9.
Jacobs F, Wisse E, De Geest B. The role of liver sinusoidal cells in hepatocyte-directed gene transfer. Am J Pathol. 2010;176:14–21.
Kwok KY, Adami RC, Hester KC, Park Y, Thomas S, Rice KG. Strategies for maintaining the particle size of peptide DNA condensates following freeze-drying. Int J Pharm. 2000;203:81–8.
Lei Y, Huang S, Sharif-Kashani P, Chen Y, Kavehpour P, Segura T. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials. 2010;31:9106–16.
Dash PR, Read ML, Barrett LB, Wolfert MA, Seymour LW. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 1999;6:643–50.
Oupický D, Koňák Č, Dash PR, Seymour LW, Ulbrich K. Effect of albumin and polyanion on the structure of DNA complexes with polycation containing hydrophilic nonionic block. Bioconjug Chem.1999;10:764–72.
Kizzire K, Khargharia S, Rice KG. High-affinity PEGylated polyacridine peptide polyplexes mediate potent in vivo gene expression. Gene Ther. 2013;20:407–16.
Khargharia S, Kizzire K, Ericson MD, Baumhover NJ, Rice KG. PEG length and chemical linkage controls polyacridine peptide DNA polyplex pharmacokinetics, biodistribution, metabolic stability and in vivo gene expression. J Control Release. 2013;170:325–33.
Allen RJ, Mathew B, Rice KG. PEG-peptide inhibition of scavenger receptor uptake of nanoparticles by the liver. Mol Pharm. 2018;15:3881–91.
Baumhover NJ, Duskey JT, Khargharia S, White CW, Crowley ST, Allen RJ, et al. Structure–activity relationship of PEGylated polylysine peptides as scavenger receptor inhibitors for non-viral gene delivery. Mol Pharm. 2015;12:4321–28.
Khargharia S, Baumhover NJ, Crowley ST, Duskey J, Rice KG. The uptake mechanism of PEGylated DNA polyplexes by the liver influences gene expression. Gene Ther. 2014;21:1021–8.
Millili PG, Yin DH, Fan H, Naik UP, Sullivan MO. Formulation of peptide nucleic acid based nucleic acid delivery construct. Bioconjug Chem. 2010;21:445–55.
Ho YP, Grigsby CL, Zhao F, Leong KW. Tuning physical properties of nanocomplexes through microfluidics-assisted confinement. Nano Lett. 2011;11:2178–82.
Lu M, Ho YP, Grigsby CL, Nawaz AA, Leong KW, Huang TJ. Three-dimensional hydrodynamic focusing method for polyplex synthesis. ACS Nano. 2014;8:332–9.
Grigsby CL, Ho YP, Lin C, Engbersen JF, Leong KW. Microfluidic preparation of polymer-nucleic acid nanocomplexes improves nonviral gene transfer. Sci Rep. 2013;3:3155.
Santos JL, Ren Y, Vandermark J, Archang MM, Williford JM, Liu HW, et al. Continuous production of discrete plasmid DNA-polycation nanoparticles using flash nanocomplexation. Small. 2016;12:6214–22.
Terebesi J, Kwok KY, Rice KG. Iodinated plasmid DNA as a tool for studying gene delivery. Anal Biochem. 1998;263:120–3.
Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–66.
Rettig, GR; Rice, KG. Quantitative in vivo imaging of non-viral-mediated gene expression and RNAi-mediated knockdown. Bioluminescence. 2009;574:155–71.
Liu Y, Berrido AM, Hua ZC, Tse-Dinh YC, Leng F. Biochemical and biophysical properties of positively supercoiled DNA. Biophys Chem. 2017;230:68–73.
Driessen RP, Sitters G, Laurens N, Moolenaar GF, Wuite GJ, Goosen N, et al. Effect of temperature on the intrinsic flexibility of DNA and its interaction with architectural proteins. Biochemistry. 2014;53:6430–8.
Wakelin LP, Bu X, Eleftheriou A, Parmar A, Hayek C, Stewart BW. Bisintercalating threading diacridines: relationships between DNA binding, cytotoxicity, and cell cycle arrest. J Med Chem. 2003;46:5790–802.
Adami RC, Collard WT, Gupta SA, Kwok KY, Bonadio J, Rice KG. Stability of peptide-condensed plasmid DNA formulations. J Pharm Sci. 1998;87:678–83.
McKenzie DL, Kwok KY, Rice KG. A potent new class of reductively activated peptide gene delivery agents. J Biol Chem. 2000;275:9970–7.
Brady J, Radonovich M, Thoren M, Das G, Salzman NP. Simian virus 40 major late promoter: an upstream DNA sequence required for efficient in vitro transcription. Mol Cell Biol. 1984;4:133–41.
Holman GG, Zewail-Foote M, Smith AR, Johnson KA, Iverson BL. A sequence-specific threading tetra-intercalator with an extremely slow dissociation rate constant. Nat Chem. 2011;3:875–81.
Millili PG, Selekman JA, Blocker KM, Johnson DA, Naik UP, Sullivan MO. Structural and functional consequences of poly(ethylene glycol) inclusion on DNA condensation for gene delivery. Microsc Res Tech. 2010;73:866–77.
Acknowledgements
The authors gratefully acknowledge the support from NIH Grants GM117785 and T32 GM00865 and for the technical support from the Central Microscopy Research Facility at the University of Iowa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mathew, B., Ramanathan, R., Delvaux, N.A. et al. Heat-shrinking DNA nanoparticles for in vivo gene delivery. Gene Ther 27, 196–208 (2020). https://doi.org/10.1038/s41434-019-0117-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0117-0